ABSTRACT
Evidence indicates that aerobic performance is degraded either by environmental heat stress or sleep deprivation. However, whether these conditions interact to produce more significant performance impairment deserves further investigation. Therefore, this study investigated the effects of experimental sleep deprivation (24 h or 96 h) on aerobic performance and thermoregulatory responses in rats exercised on a treadmill at different environmental conditions. Adult male Wistar rats were subjected to rapid eye movement sleep deprivation (RSD) using the modified multiple platform method and were then subjected to an incremental-speed exercise until they were fatigued. Treadmill running was performed in a temperate (24°C) or warm (31°C) environment, and the colonic temperature (an index of core body temperature; TCORE) and the tail-skin temperature (TSKIN; an index of cutaneous heat loss) were recorded. 24-h and 96-h RSD produced small magnitude reductions in aerobic performance (Cohen’s d = 0.47–0.58) and minor changes in thermoregulation. Relative to control rats, sleep-deprived rats showed a higher TCORE at the exercise initiation and a higher threshold for activating cutaneous heat loss, but unchanged TCORE and TSKIN at fatigue. Exercise at 31°C induced large reductions in performance (d = 0.82–1.29) and marked changes in thermoregulation, as evidenced by higher TCORE and TSKIN at fatigue, compared to exercise at 24°C. Interestingly, none of the effects induced by RSD were exacerbated by environmental heat stress and vice-versa, indicating that both conditions did not interact. We conclude that RSD and heat stress modulate aerobic performance and thermoregulatory responses by acting independently.
Introduction
Sleep deprivation is a common issue in modern societies [Citation1], and its prevalence has been increasing due to constant exposure to artificial light and interactive activities, which combine with social and economic pressures to shorten the time spent asleep [Citation2]. Sleep deprivation can be divided into conditions of complete (such as in ultra-endurance sporting events and military exercises) or partial deprivation (such as in individuals experiencing sleep disorders or flying across time zones, and in shift workers) [Citation3]. Affected individuals usually report feelings of persistent tiredness and lack of energy [Citation2,Citation3].
In the sports context, sleep disruption in athletes often occurs due to rapid travel across multiple meridians, anxiety before important events, and training and competition schedules [Citation4–6]. A study with a large number of nationally-ranked athletes revealed they only sleep between 6 and 7 h per night [Citation7]. Evidence suggests that reduced sleep is associated with altered neurotransmission [Citation8,Citation9] and neurobehavioral deficits in daytime performance [Citation7]; therefore, the level of sleep loss commonly observed in athletes could be associated with impaired performance and recovery.
Previous studies have reported reduced aerobic performance following total sleep deprivation in humans [Citation10–12], although this is not a universal observation [Citation13]. Because aerobic performance is modulated by thermoregulation, there are investigations about the impact of sleep deprivation on body temperatures in individuals subjected to physical exercise. A lower core body temperature (TCORE) was observed during exercise in sleep-deprived subjects [Citation12], even though a tendency for enhanced exercise-induced increases in TCORE was also reported [Citation14]. Noteworthy, most of the studies on the topic have failed to notice differences in the increase in TCORE induced by cycling [Citation15,Citation16] or running [Citation17].
In the laboratory, experimental models have been developed for studying the consequences of sleep deprivation in rats. This study will focus on repeated eye movement (REM) sleep, which is assumed to play a role in restoring some deficits incurred during prior wakefulness and preparing for ensuing wakefulness [Citation18]. In this sense, REM sleep deprivation (RSD) adversely affects several body functions, including food intake, energy metabolism, body weight [Citation19], and the regulation of TCORE. Several authors demonstrated that total sleep deprivation and RSD modify the thermoregulatory responses in rats, with either report of hypothermia [Citation20–24] or hyperthermia [Citation20,Citation21,Citation23–25]. Of note, the predominant thermoregulatory manifestation depends on the duration of sleep deprivation: the initial hyperthermia is usually replaced by hypothermia as deprivation progresses and the risk of dying increases [Citation20,Citation21,Citation23,Citation24]. Because increased TCORE at exercise initiation reduces aerobic performance, particularly in the heat [Citation26,Citation27], rats subjected to short-term RSD (i.e., 24 h or 96 h) would likely fatigue earlier than control rats.
Like sleep deprivation, warm/hot environments impact aerobic performance and thermoregulatory responses during prolonged exercise [Citation26,Citation28,Citation29]. For example, rats exhibit greater increases in brain, abdominal, and tail-skin (TSKIN) temperatures [Citation26,Citation30,Citation31], greater cardiovascular strain [Citation32,Citation33], and reduced aerobic performance [Citation26,Citation30–32] while exercising in warm/hot conditions compared with temperate conditions. Running in the heat also changes neurotransmission in rats, as indicated by higher dopamine and noradrenaline concentrations in the preoptic area and anterior hypothalamus [Citation34]. Therefore, because RSD and environmental heat stress influence neurotransmission, it is likely that the performance and thermoregulatory impairments induced by these two factors share common pathways, so that the effects mediated by RSD would be exacerbated in a warm environment.
Thus, the present study aimed to evaluate the effects of either 24-h or 96-h RSD on aerobic performance and thermoregulatory responses in rats subjected to an exercise session at different environmental conditions. Considering that TCORE levels at exercise initiation modulate aerobic performance, we hypothesized that sleep-deprived rats would exhibit higher initial TCORE and altered thermoregulatory control during exercise that would accelerate fatigue. We also expected that the thermoregulatory and performance impairments would be more evident in sleep-deprived rats under environmental heat stress conditions.
Materials and methods
Animals
Adult male Wistar rats weighing 250–350 g were used in the experiments. The rats were housed in collective polypropylene cages (3 animals per cage), under controlled light (lights on from 7:00 am until 7:00 pm) and temperature (24 ± 1°C) conditions, with water and rat chow provided ad libitum. All experimental procedures were approved by the local ethics commission for the use of animals (protocol 273/2014) and were performed following the regulations provided by the National Council for the Control of Animal Experimentation (CONCEA/Brazil).
Experimental design
The study was divided into four experiments (), and different rats were used in each experiment. The first experiment aimed to investigate the effects of a 24-h RSD on thermoregulatory responses and aerobic performance of rats subjected to an incremental-speed fatiguing exercise in a temperate environment (24°C). Moreover, the experiment 1 also aimed to investigate whether our control conditions (rats kept on a grid where they could sleep) represented an adequate control trial indeed. Therefore, the thermoregulation and performance of rats kept on the grid (without any contact with cedar-wood shavings) were compared to the responses of rats kept in their home cages filled with cedar-wood shavings. In this first set of experiments, nine rats were subjected to three trials: (I) home cage-control – rats that were not subjected to the RSD protocol and that could sleep in their home cages on cedar-wood shavings; (II) control – rats that were not subjected to the RSD protocol and that could sleep on the grid; (III) sleep deprivation – rats that were subjected to the RSD protocol. This protocol (or the control procedure) was always initiated at 7:00 am and ended at 7:00, 8:00, or 9:00 pm on the same day, when the exercise session was initiated. A minimum interval of 72 h separated the exercise sessions, and the order of the trials was balanced, as defined by a Latin square design.
Figure 1. Experimental designs. * indicates the days at which the incremental-speed exercises were carried out. Legend: Exp, experiment; RSD, rapid eye movement sleep deprivation
The second experiment investigated the effects of 24-h RSD on thermoregulation and performance of rats subjected to incremental exercises in a warm environment (31°C). Nine animals were subjected to two trials: (I) control and (II) sleep deprivation. During the experimental trials, the procedures were similar to those adopted in the first set of experiments, except for the ambient temperature and the inexistence of a control trial with rats kept in their home cages. The order of the trials was randomized.
The third experiment investigated the effects of 96-h RSD on thermoregulation and performance of rats subjected to incremental exercises in a temperate environment. Nine rats were subjected to two trials (i.e., control and sleep deprivation). The RSD protocol was always initiated at 7:00 am and ended at 7:00, 8:00, or 9:00 pm on the fourth day, when the exercise sessions were initiated. A minimum interval of 120 h separated the exercise sessions, and the order of the trials was randomized. The fourth experiment was identical to experiment 3, except the ambient temperature at which treadmill running was carried out (i.e., 31°C, warm environment).
In all experiments, exercise was performed at the transition between the light (inactive) and dark (active) phases when rats are expected to wake up. We also avoided subjecting the same rat to two RSD procedures, because as the animals are subjected to more days into a sleep-deprived state, they became more adapted to this condition, possibly changing their sleep profile over time [Citation35]. Moreover, following the last trial, the rats were euthanized with an intraperitoneal injection of a lethal dose of anesthetic (ketamine 240 mg/kg and xylazine 31.5 mg/kg).
Familiarization with the experimental procedures
The familiarization protocol was conducted across five consecutive days. Light electrical stimulation (0.5 mA) was applied to gradually encourage the rats to exercise on a treadmill designed for small animals (Panlab, Harvard Apparatus, Spain). In each daily session, after resting for 5 min on the treadmill belt, the rats were required to run for 5 min at a constant speed (11 m/min on the first day, with a daily increase of 1-m/min) and a constant incline of 5%. In the last three familiarization sessions, the rats run with a thermistor inserted into their colon and a thermocouple attached to the lateral surface close to the base of their tail. The purpose of these preliminary exercise sessions was to show the rats the direction they should run without becoming entangled in the thermistor/thermocouple wires, thereby allowing for consistent treadmill performance and minimizing their exposure to electrical stimuli during the experimental trials [Citation36,Citation37]. Animals were also familiarized with the sleep deprivation procedure for 60 min during these five consecutive days.
RSD protocol
Sleep deprivation was accomplished by using the modified multiple platform method proposed by Suchecki [Citation38]. This method is known as an inducer of RSD (or paradoxical sleep deprivation, as commonly used in the past), although it also produces partial deprivation of slow-wave sleep [Citation39]. Seven platforms (6 cm in diameter each) were placed inside a big polypropylene cage (56 x 36 × 21 cm) filled with water. Three rats, always taken from the same home cage in the vivarium, were placed in a cage with multiple platforms to prevent social instability from enhancing stress [Citation38]. The rats were placed on top of the platforms so that they could move around. The cages were filled with water until 1 cm below the top of the platforms, and the water was changed daily. Because of atonia in the antigravity muscles at the onset of REM sleep, the rats on the small platforms tended to fall into or touch the surrounding water, resulting in REM sleep loss [Citation24]. Exposure to the multiple platforms began at 7:00 am and lasted for 12 h or 84 h.
Control experiments were performed in the same big polypropylene cages placed in the same room as the sleep-deprived rats for the duration of the procedure. However, the control rats were placed on a metallic grid floor, with water until 1 cm below the floor. The rats were allowed to lie down without falling into the water, albeit their tails might have touched the water [Citation38]. The grid was made of stainless steel, with the rods placed 2.3 cm apart from each other.
The room where RSD and control protocols were carried out was maintained under controlled light (lights on from 7:00 am until 7:00 pm) and temperature (24 ± 1°C) conditions, which were similar to the conditions maintained at the vivarium. Food and water were provided ad libitum.
Physical exercise trials
The rats were always subjected to the exercise trials at 7:00, 8:00, or 9:00 pm (beginning of the dark phase). On the day when experiments were conducted, each rat was weighed, a thermistor (MEAS 4400, Dayton, OH, USA) was inserted 7 cm beyond the anal sphincter to measure colonic temperature (an index of TCORE), and a thermocouple was attached to the lateral surface, 1 cm from the base of the tail (Instrutherm, model S-09 K, Brazil) to measure TSKIN. The close proximity to the base of the tail enabled more sensitive measurements of changes in TSKIN that occurred as a function of changes in local blood flow [Citation40]. Notably, the TSKIN in heat-exchange organs, such as the rat tail, primarily depends on the local vasomotor tone and is not a reliable measure of ambient temperature [Citation41].
The rats were then subjected to an incremental speed exercise (starting at a speed of 13 m/min, with 1.3 m/min increases every 3 min). The exercise was performed until volitional fatigue, which was defined as when the rats were no longer able to keep pace with the treadmill for 10 s, even when stimulated by light electrical shocks [Citation36,Citation42].
The TCORE and TSKIN were recorded every 60 s throughout the exercise sessions, and the time to fatigue was considered a physical (aerobic) performance index.
Ambient temperature control
Ambient temperature (TAMB) was measured inside the chamber that involves the treadmill belt with two thermocouples, one positioned in the front and the other in the back of the treadmill. In the experiments performed under temperate conditions, TAMB was controlled at 24°C with air-conditioning. This TAMB was selected as a temperate environment because previous data suggest that TAMB ranging from 24 to 26°C corresponds to the lower extremity of the thermoneutral zone of resting rats maintained inside the chamber that contained the treadmill belt [Citation37,Citation43,Citation44]. In the warm experiments, the TAMB was controlled at 31°C by using two heaters turned on at 1,200 W (Britânia model AB 1100, Curitiba, Brazil), one positioned in the front and the other in the back of the treadmill, in order to avoid the surging of a temperature gradient in the chamber. TAMB was recorded every 60 s throughout the exercise sessions.
Calculations
To identify differences in the central modulation of thermoeffector activity, the TCORE threshold for cutaneous heat loss was calculated using a method based on that described by Cheuvront et al. [Citation45]. This method has been used to investigate thermoregulatory responses in rats [Citation31,Citation37]. Briefly, the TSKIN was plotted against TCORE, and then two independent investigators identified the threshold for activation of tail heat loss visually; a consensus-derived value was used. Next, the data before and after this threshold were separated. Linear regression analyses were performed for data before and after the threshold to describe the relationship between TSKIN and TCORE. The intersection of the regression lines was used to determine the heat loss threshold. At this moment, we could objectively verify whether visual identification of the threshold was correct. If not, the analysis was redone from the beginning.
Statistical analysis
Shapiro-Wilk’s and Levene’s tests were used to assess the normality and homoscedasticity of the data. Because all data presented a normal distribution, they were expressed as means ± standard errors of the mean (SEM). In experiment 1, TCORE and TSKIN were compared across exercise time points and between experimental trials (home cage vs. grid) using two-way ANOVAs with repeated measures. When significant main effects or interactions were observed, these ANOVAs were followed by Tukey’s post hoc tests to identify differences between pairs of means. The time to fatigue, TCORE, and TSKIN at exercise initiation and fatigue, exercise-induced changes in TCORE, and the threshold for cutaneous heat loss were compared between trials using paired Student’s t-tests.
In the other experiments, TCORE and TSKIN were compared across exercise time points and between treatments (RSD vs. control) and TAMB (24°C vs. 31°C) using three-way ANOVAs. Moreover, the seven parameters mentioned above were compared between treatments and TAMB using two-way ANOVAs, followed by Tukey’s tests.
The significance level was set at α < 0.05. Cohen’s d effect size (ES) was calculated to assess the magnitude of the differences in data between groups. The ES was calculated by subtracting the mean value for one group from the mean value of the group it was being compared to. The result was then divided by a combined standard deviation for the data. The ES values were classified as trivial (< 0.2), small (0.2–0.6), moderate (0.6–1.2), large (1.2–2.0), very large (2.0–4.0), and extremely large (> 4.0) [Citation46].
Results
Selection of the setup for experiments in control rats
The rats that were kept in their home cages ran for 41.6 ± 2.6 min, which means that on average, they almost have completed the 14th stage of the incremental exercise performed at 24°C. When the rats were kept on the grid (but not subjected to RSD), they displayed similar physical performance relative to the trial when they were kept in their home cages ().
Table 1. Physical performance and thermoregulatory responses in control rats either kept in their home cages or on the grid and then subjected to an incremental exercise
The rats had similar TCORE at the beginning of exercise, irrespective of whether they were kept (). Treadmill running markedly increased TCORE in the two experimental trials, so that rats fatigued with temperature values above 40.0°C (Supplementary Figure 1A). Although a trial × time point interaction was observed, the post hoc test failed to identify differences between pairs of means. Moreover, no differences in TCORE were observed between trials at fatigue ().
At the beginning of exercise, the rats had lower TSKIN when kept on the grid than when kept in their home cages (); the difference between trials corresponded to a very large effect size. In both trials, TSKIN transiently decreased in response to exercise initiation until reaching a nadir at the 4th min of running (Supplementary Figure 1B). The reduction in TSKIN was followed by a gradual increase that was observed until this temperature has reached a plateau; after that, TSKIN remained elevated (around 33°C) until the rats had fatigued. The lower initial TSKIN values observed in rats kept on the grid persisted until the 9th min of exercise. From then on, including at fatigue, no differences between trials were observed ().
Because the lack of bedding influenced TSKIN, particularly at the beginning of exercise, we decided to maintain the control rats on the grid in the subsequent experiments.
Experiments after 24-h RSD or the control procedure
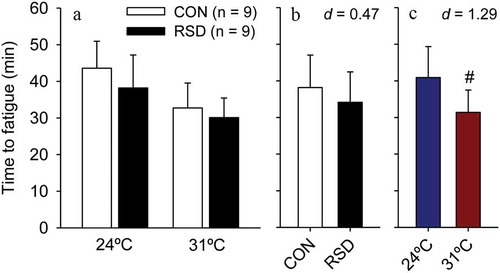
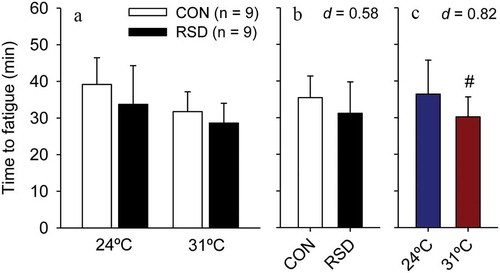
The analyses of time to fatigue in rats subjected to 24-h RSD or the control procedure indicated that the main effect of treatment did not reach statistical significance (F = 2.76; p = 0.106; ); indeed, RSD produced only a small magnitude effect in physical performance (ES = 0.47). In contrast, a significant main effect of ambient temperature was observed (F = 15.39; p < 0.001), with rats running 23% less at 31°C than at 24°C; the warmth-induced degradation of aerobic performance was classified as large effect size (ES = 1.29). Of note, no interaction was observed between these two factors (F = 0.33; p = 0.570), indicating that the effects of 24-h RSD on time to fatigue did not depend on the ambient temperature and vice-versa.
Figure 2. Time to fatigue in rats subjected to 24-h rapid eye movement sleep deprivation (RSD) or the control procedure; physical exercise was performed at two ambient temperatures: 24°C or 31°C (panel A). The data are expressed as means ± SEM and were analyzed using two-way ANOVAs, followed by Tukey’s post hoc test. # denotes a significant (p < 0.05) main effect of ambient temperature. Panel B shows a small reduction in time to fatigue caused by RSD, irrespective of the ambient temperature. Panel C shows a large reduction in time to fatigue caused by the warm environment, irrespective of whether rats were or were not subjected to RSD
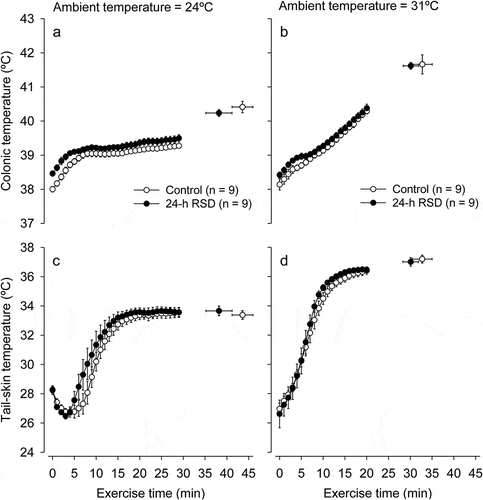
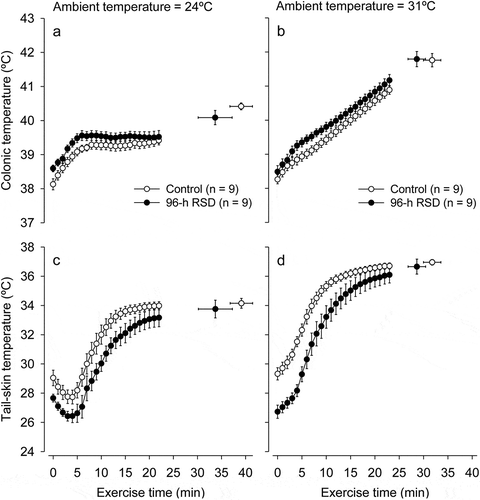
Treadmill running induced the expected increases in TCORE and TSKIN in all experimental trials. The increases in TCORE and TSKIN were more accentuated at 31°C than at 24°C; also, the increase in TSKIN was observed after a short-latency period at 24°C but not at 31°C (). We then performed additional analyses to better understand the influence of RSD and ambient temperature in TCORE and TSKIN at specific time-points (i.e., the beginning of exercise or at fatigue), as well as in the exercise-induced changes in these body temperatures. As detailed below, the exercise-induced changes in TCORE were influenced by both the 24-h RSD and the warm environment, whereas the changes in TSKIN were mainly influenced by the environment.
Figure 3. Changes in colonic temperature (panels A and B) and tail-skin temperature (panels C and D) induced by physical exercise at 24°C or 31°C in control rats (white circles) or rats subjected to 24-h rapid eye movement sleep deprivation (RSD, black circles). The temperature data are shown until the exercise time point when the first rat stopped running, whereas the scatter plots with bi-directional error bars indicate the temperatures measured at fatigue. The data are expressed as means ± SEM and were analyzed using three-way ANOVAs. These analyses yielded the following significant results, which are similar for both panels: treatment effect (p < 0.001) and interaction between the ambient temperature × time (p < 0.001). No other significant interactions were observed
24-h RSD increased the rats’ TCORE at exercise initiation (F = 11.65; p = 0.002), but did not change this temperature at fatigue (F = 0.28; p = 0.603; , thus indicating the existence of a reduced exercise-induced change in TCORE in sleep-deprived rats (F = 6.29; p = 0.017; . The RSD-induced effects in initial TCORE (a 0.37°C increase) and in the TCORE change (a 0.48°C reduction) were classified as moderate and small effect sizes, respectively (ES = 1.15 and 0.56). These sleep-deprived rats did not present altered cutaneous heat loss, as indicated by the lack of a treatment effect in TSKIN at exercise initiation (F = 0.08; p = 0.775) and at fatigue (F = 0.03; p = 0.876; . However, 24-h RSD produced a moderate increase in the threshold TCORE for cutaneous heat loss (F = 4.55; p = 0.041; ES = 0.68; .
Figure 4. The colonic temperature at fatigue (panel A), exercise-induced changes in colonic temperature (panel D), the tail-skin temperature at fatigue (panel G), and colonic temperature threshold for cutaneous heat loss (panel J) in rats subjected to 24-h rapid eye movement sleep deprivation (RSD) or the control procedure; physical exercise was performed at two ambient temperatures: 24°C or 31°C. The data are expressed as means ± SEM and were analyzed using two-way ANOVAs, followed by Tukey’s post hoc test. + denotes a significant (p < 0.05) main effect of experimental group. # denotes a significant (p < 0.05) main effect of ambient temperature. The panels B, E, H, and K show the effect sizes of the changes induced by RSD, irrespective of the ambient temperature. The panels C, F, I, and L show the effect sizes of changes induced by the warm environment, irrespective of whether rats were or were not subjected to RSD
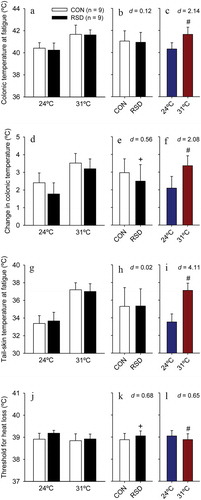
The warm environment did not influence TCORE (F = 0.19; p = 0.666) but influenced TSKIN (F = 7.21; p = 0.011) at exercise initiation. This result (i.e., a − 1.49°C reduction in TSKIN at 31°C; large effect) is unexpected because both the exercise and heat exposure were initiated simultaneously; therefore, the exposure was not sufficiently long to change thermoregulatory parameters. As expected, the warm environment exaggerated the exercise-induced increases in TCORE () and TSKIN, with both values being higher at fatigue during experiments performed at 31°C than at 24°C (F = 39.61 and p < 0.001 for TCORE; F = 146.09 and p < 0.001 for TSKIN; . These heat-induced greater body temperatures at fatigue were classified as very large effect sizes (ES > 2.10). The warm environment also induced a moderate reduction in the threshold TCORE for cutaneous heat loss (F = 4.15; p = 0.050; ES = 0.65; ).
Interestingly, as previously reported for physical performance, no interactions were observed between 24-h RSD and ambient temperature in any of the thermoregulatory responses determined in running rats, with p-values higher than 0.230 for each of the six responses evaluated (i.e., TCORE at exercise initiation and fatigue, exercise-induced changes in TCORE, TSKIN at exercise initiation and fatigue, and the threshold for cutaneous heat loss).
Experiments after 96-h RSD or control procedure
The results observed following 96-h RSD were quite similar to those observed following 24-h RSD. The time to fatigue analysis indicated that 96-h RSD only produced a small (ES = 0.58), not statistically significant, reduction in physical performance (F = 2.95; p = 0.096; ). In contrast, the main effect of ambient temperature was observed, with rats running for shorter periods at 31°C than at 24°C (F = 6.25; p = 0.018); the average 6-min reduction in time to fatigue induced by the warm environment was classified as moderate effect size (ES = 0.82). No interaction was observed between these two factors (F = 0.21; p = 0.652), indicating that the effects of 96-h RSD on time to fatigue did not depend on the ambient temperature and vice-versa.
Figure 5. Time to fatigue in rats subjected to 96-h rapid eye movement sleep deprivation (RSD) or the control procedure; physical exercise was performed at two ambient temperatures: 24°C or 31°C (panel A). The data are expressed as means ± SEM and were analyzed using two-way ANOVAs, followed by Tukey’s post hoc test. # denotes a significant (p < 0.05) main effect of ambient temperature. Panel B shows a small reduction in time to fatigue caused by RSD, irrespective of the ambient temperature. Panel C shows a large reduction in time to fatigue caused by the warm environment, irrespective of whether rats were or were not subjected to RSD
As observed for the experiments after 24-h RSD, treadmill running induced the expected increases in TCORE and TSKIN in all trials performed after 96-h RSD, with these changes being more accentuated at 31°C than at 24°C (). Although a significant ambient temperature × treatment interaction was reported for TSKIN (F = 4.01, p = 0.014), the post hoc analysis could not identify why the effect of ambient temperature on TSKIN would be dependent on the treatment evaluated.
Figure 6. Changes in colonic temperature (panels A and B) and tail-skin temperature (panels C and D) induced by physical exercise at 24°C and 31°C in control rats (white circles) or rats subjected to 96-h rapid eye movement sleep deprivation (RSD, black circles). The temperature data are shown until the exercise time point when the first rat stopped running, whereas the scatter plots with bi-directional error bars indicate the temperatures measured at fatigue. The data are expressed as means ± SEM and were analyzed using three-way ANOVAs. These analyses yielded the following significant results, which are similar for both panels: treatment effect (p < 0.001) and interaction between the ambient temperature × time (p < 0.001). A significant interaction between ambient temperature × treatment (p = 0.014) was only observed for tail-skin temperature. No other significant interactions were observed
96-h RSD increased the rat’s TCORE at exercise initiation (F = 5.631; p = 0.024) but did not change this temperature at fatigue (F = 0.624; p = 0.435; , thus indicating the existence of a reduced exercise-induced change in TCORE in sleep-deprived rats (F = 5.568; p = 0.025; . The RSD-induced effects in initial temperature (a 0.34°C increase) and in the TCORE change (a 0.49°C reduction) were classified as moderate and small magnitude effect sizes (ES = 0.78 and 0.56, respectively). Sleep-deprived rats presented lower TSKIN at exercise initiation (F = 21.639; p < 0.001), a large effect mediated by 96-h RSD (ES = 1.34) that contrasts with results following 24-h RSD. Additionally, 96-h RSD did not influence the TSKIN value at fatigue (F = 0.607; p = 0.442; but produced a large increase in the threshold TCORE for cutaneous heat loss (F = 18.839; p < 0.001; ES = 1.45; .
Figure 7. The colonic temperature at fatigue (panel A), exercise-induced changes in colonic temperature (panel D), the tail-skin temperature at fatigue (panel G), and colonic temperature threshold for cutaneous heat loss (panel J) in rats subjected to 96-h rapid eye movement sleep deprivation (RSD) or the control procedure; physical exercise was performed at two ambient temperatures: 24°C or 31°C. The data are expressed as means ± SEM and were analyzed using two-way ANOVAs, followed by Tukey’s post hoc test. + denotes a significant (p < 0.05) main effect of experimental group. # denotes a significant (p < 0.05) main effect of ambient temperature. The panels B, E, H, and K show the effect sizes of changes induced by RSD, irrespective of the ambient temperature. The panels C, F, I, and L show the effect sizes of changes induced by the warm environment, irrespective of whether rats were or were not subjected to RSD
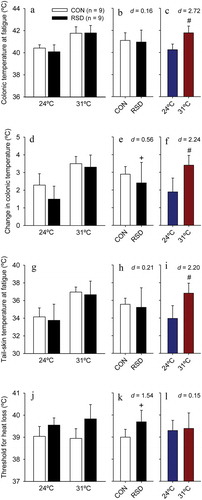
As expected, the warm environment exaggerated the exercise-induced increases in TCORE () and TSKIN, with both values being higher at fatigue during experiments at 31ºC than at 24ºC (F = 65.490 and p < 0.001 for TCORE; F = 41.661 and p < 0.001 for TSKIN; . These heat-induced greater body temperatures at fatigue were classified as very large effect sizes (ES > 2.20). No significant effects mediated by the warm environment were observed in the TCORE (F = 0.008; p = 0.931) and TSKIN (F = 0.587; p = 0.449) at exercise initiation and in the threshold TCORE for cutaneous heat loss (F = 0.347; p = 0.560; ).
Again, no interactions were observed between 96-h RSD and ambient temperature in the thermoregulatory responses determined in running rats, with p-values higher than 0.210 for each of the six responses evaluated.
Discussion
This study investigated the effects of RSD, environmental heat stress, and the interaction between them on aerobic performance and exercise-induced changes in thermoregulatory responses. More specifically, this investigation was performed in rats subjected to two different durations of RSD (i.e., 24 h and 96 h). Our findings revealed that 24-h RSD produced a small magnitude effect on aerobic performance and modified thermoregulatory responses; a greater TCORE was observed at exercise initiation but not at fatigue, thereby indicating a lower running-induced increase in TCORE. Cutaneous heat loss through tail vessels was barely affected in sleep-deprived rats. Quite similar effects on physical performance and thermoregulatory responses were detected following the more prolonged RSD protocol (i.e., 96 h). As expected, environmental heat stress increased TCORE and TSKIN values observed at fatigue and markedly reduced aerobic performance. More relevant, the performance and thermoregulatory effects mediated by RSD and environmental heat stress were, in all cases, independent from each other.
In the present study, small decreases in performance were reported in 24-h and 96-h sleep-deprived rats. This is a novel finding, as no previous studies have addressed the association between aerobic performance and RSD in laboratory animals. Several studies indicate that total sleep deprivation reduces performance in humans [Citation10–12]. For instance, Martin [Citation10] observed an 11% reduction in time to exhaustion during prolonged treadmill walking (80% VO2max) following a 36-h sleep deprivation period, while Martin & Cheng [Citation11] observed a 20% reduced time to exhaustion during constant-intensity walking protocol following 50 h of sleep deprivation. Using a more ecologically valid protocol to assess athletes’ performance, Oliver et al. [Citation12] observed a reduction in the distance covered during 30 min of self-paced treadmill running following one night of sleep deprivation. These studies suggest that the degraded aerobic performance caused by sleep deprivation might be attributed to changes in the brain, as limited effects were observed in cardiorespiratory or thermoregulatory functions [Citation12] and the peripheral sympathetic nervous system [Citation11]. The observation that subjects ran less distance after sleep deprivation than after a typical night but with a similar rating of perceived exertion in both conditions also supports the determinant role of central mechanisms [Citation12].
Evidence indicates that RSD influences the production and release of neurotransmitters such as dopamine, noradrenaline, and acetylcholine [Citation2,Citation8,Citation9,Citation47]. Since these neurotransmitter systems’ activity has been implicated in the modulation of aerobic performance [Citation42,Citation48,Citation49], we hypothesized that RSD-induced changes in neurotransmitter levels would impair performance. In fact, RSD reduces dopamine levels without affecting serotonergic transmission in the striatum [Citation9]. Considering that mice selectively bred for high rates of voluntary exercise on running wheels have higher basal levels of DA in the caudate-putamen (i.e., dorsal striatum) [Citation50,Citation51], it is suggestive that reduced DA in the striatum is one of the central factors underlying decreased performance caused by RSD.
Regarding the thermoregulatory responses, RSD rats presented greater TCORE at exercise initiation, a finding that agrees with other studies, in which short-term REM/total sleep deprivation increased the TCORE of the animals [Citation23–25]. Jaiswal et al. [Citation24] observed marked hyperthermia (~1.2°C rise) already on the 1st day of RSD; this hyperthermia was then gradually reversed with rats presenting normal TCORE levels within 6–8 days of RSD. Interestingly, RSD was reported to increase noradrenaline both in the blood [Citation20] and in the brain [Citation48], and the RSD-induced hyperthermic response was blocked after systemic administration of prazosin, an alpha-1 adrenoceptor antagonist. Thus, according to Jaiswal et al. [Citation24], the increased TCORE in sleep-deprived rats results from augmented sympathetic outflow that induces peripheral vasoconstriction and increases metabolism.
The higher initial TCORE in our sleep deprived-rats does not agree with findings in humans. Total sleep deprivation has induced either decreased [Citation12,Citation52] or unaltered baseline TCORE [Citation14] in humans, whereas REM sleep deprivation produced hypothermia in the first recovery night after deprivation [Citation53]. The discrepancy in the thermoregulatory response between the two species (i.e., humans and rats) may result from the fact that sleep deprivation protocols are attained voluntarily in humans (which are aware of potential adverse effects) but involuntarily in rats. Thus, sleep deprivation protocols are likely more stressful in laboratory rodents than in humans. Elevated TCORE is a common feature that has been reported in rats subjected to several experimental models for inducing psychological stress, such as the cage-switch stress [Citation54], handling [Citation55], and expectation for exercise initiation after being placed on the treadmill belt [Citation56].
Exercise fatigue has been accepted as a brain-mediated process that depends on multiple afferent signals [Citation57]. This afferent (sensory) feedback includes high TCORE levels, a limiting factor for aerobic performance [Citation26,Citation27,Citation58,Citation59]. In particular, higher TCORE values at exercise initiation have been associated with a lower aerobic performance in rats [Citation26,Citation60,Citation61], although this is not a universal finding [Citation56]. In the present study, despite the fact that RSD rats presented greater TCORE at exercise initiation and a higher heat loss threshold than control rats, no differences in TCORE were observed at fatigue. Moreover, these findings mean that sleep deprived-rats presented lower exercise-induced changes in TCORE, likely resulting from augmented heat loss or reduced metabolic rate during treadmill running.
The hypothesis of augmented heat loss through tail vessels seems unlike, however. As indicated by TSKIN values, cutaneous heat loss was barely affected by 24-h RSD; a higher TCORE threshold for cutaneous heat loss was observed in sleep-deprived rats, even though this effect did not lead to greater TCORE values at fatigue. The more prolonged RSD (i.e., 96 h) reduced TSKIN at exercise initiation, but this response was no longer observed as rats were exercised. Because tail vasodilation is the main pathway by which rats dissipate body heat while running [Citation44,Citation62,Citation63], alternative and maybe “underestimated” pathways for dissipating heat (e.g., evaporative breathing) may help explain the lower exercise-induced increase in TCORE in sleep-deprived rats. Interestingly, a recent study failed to report changes in TCORE during exercise, despite an apparent attenuation in the TSKIN increase induced by caudal artery denervation [Citation43].
To date, no study has investigated whether oxygen consumption (VO2) is decreased in sleep-deprived rats subjected to treadmill running. However, the following two observations suggest that a lower VO2 in sleep-deprived rats is very unlikely. First, men after 30 h without sleep presented slightly increased VO2 while running at 60% of their VO2MAX [Citation12]. Second, a reduced VO2 during treadmill running would mean a decreased oxygen cost of locomotion, usually associated with increased performance, as recently observed in aerobically trained rats [Citation64].
As expected [Citation28,Citation29,Citation32], the aerobic performance was greatly impaired in the warm compared with the temperate environment, regardless of whether the rats were subjected or not to sleep deprivation. The rats presented exaggerated body hyperthermia at 31°C relative to 24°C, as evidenced by higher TCORE and TSKIN at fatigue. The reduced performance under warm conditions has been attributed to greater hyperthermia [Citation65], greater cardiovascular strain [Citation66], and altered thermal perception [Citation67]. Indeed, several psychophysiological mechanisms play a role in the association between hyperthermia and fatigue, including higher perceived exertion, reduced drive for skeletal muscle contraction, reduced cerebral blood flow, and endotoxemia [Citation65].
Sleep quality has been listed as an intrinsic modulator that changes thermoregulation and the risk of developing heat stroke [Citation68], a severe heat-related disorder [Citation69]. In the present study, RSD did not exaggerate the exercise-induced increases in TCORE, regardless of the environmental conditions. Notably, the magnitude of hyperthermia is positively associated with intestinal permeability during exercise [Citation70]. Augmented intestinal permeability allows bacterial translocation [Citation71], which may trigger a systemic inflammatory response that, under certain conditions, favors the occurrence of heatstroke [Citation72]. Although we did not measure intestinal permeability and inflammatory response, any eventual impairment in gastrointestinal function induced by sleep deprivation would likely not result from altered thermoregulatory control, at least in the experimental conditions evaluated herein.
The changes in aerobic performance and thermoregulatory responses mediated by RSD and heat exposure were always independent of each other. For example, the reduced time to fatigue observed in sleep-deprived rats was not exaggerated when rats were exercised at 31°C. These findings suggest that the two experimental manipulations produce their effects acting by independent mechanisms. This hypothesis should be addressed in future analyses regarding the neuronal activation or neurotransmitter release in brain areas modulating thermoregulation and performance. Another hypothesis to explain the lack of interactions is that the exercise under environmental heat stress might induce a marked thermoregulatory strain that would override any impairment caused by RSD. This hypothesis is supported by the observation that esophageal temperature was 0.3°C higher in individuals after 33 h of wakefulness; nevertheless, this difference was no longer observed during a subsequent exercise at 35°C [Citation16].
We conclude that RSD and heat stress induced, respectively, small and large changes in the aerobic performance of running rats, thus indicating that degraded performance was more evident in the warm environment than after RSD. Besides, both experimental manipulations influenced thermoregulation in rats, even though RSD more clearly influenced thermoregulation in non-exercise conditions than during exercise. The warm environment, as expected, exaggerated the exercise-induced hyperthermia. Finally, the present data suggest that RSD and environmental heat stress modulate performance and thermoregulation by acting through independent mechanisms.
Disclosure statement
The authors have no potential conflicts of interest to disclose.
Additional information
Funding
References
- Ford ES, Cunningham TJ, Croft JB. Trends in self-reported sleep duration among US adults from 1985 to 2012. Sleep. 2015;38:829–832.
- Tufik S, Andersen ML, Bittencourt LR, et al. Paradoxical sleep deprivation: neurochemical, hormonal and behavioral alterations. Evidence from 30 years of research. An Acad Bras Cienc. 2009;81:521–538.
- Temesi J, Arnal PJ, Davranche K, et al. Does central fatigue explain reduced cycling after complete sleep deprivation? Med Sci Sports Exercise. 2013;45(12):2243–2253.
- Juliff LE, Halson SL, Peiffer JJ. Understanding sleep disturbance in athletes prior to important competitions. J Sci Med Sport. 2015;18(1):13–18.
- Reilly T, Edwards B. Altered sleep-wake cycles and physical performance in athletes. Physiol Behav. 2007;90(2–3):274–284.
- Sargent C, Roach GD. Sleep duration is reduced in elite athletes following night-time competition. Chronobiol Int. 2016;33:667–670.
- Sargent C, Lastella M, Halson SL, et al. The impact of training schedules on the sleep and fatigue of elite athletes. Chronobiol Int. 2014;31:1160–1168.
- Mallick BN, Singh A. REM sleep loss increases brain excitability: role of noradrenaline and its mechanism of action. Sleep Med Rev. 2011;15:165–178.
- Proença MB, Dombrowski PA, Cunha CD, et al. Dopaminergic D2 receptor is a key player in the substantia nigra pars compacta neuronal activation mediated by REM sleep deprivation. Neuropharmacology. 2014;76:118–126.
- Martin BJ. Effect of sleep deprivation on tolerance of prolonged exercise. Eur J Appl Physiol Occup Physiol. 1981;47(4):345–354.
- Martin BJ, Chen HI. Sleep loss and the sympathoadrenal response to exercise. Med Sci Sports Exerc. 1984;16(1):56–59.
- Oliver SJ, Costa RJ, Laing SJ, et al. One night of sleep deprivation decreases treadmill endurance performance. Eur J Appl Physiol. 2009;107(2):155–161.
- Goodman J, Radomski M, Hart L, et al. Maximal aerobic exercise following prolonged sleep deprivation. Int J Sports Med. 1989;10(6):419–423.
- Sawka MN, Gonzalez RR, Pandolf KB. Effects of sleep deprivation on thermoregulation during exercise. Am J Physiol. 1984;246:R72–R77.
- Dewasmes G, Bothorel B, Hoeft A, et al. Regulation of local sweating in sleep-deprived exercising humans. Eur J Appl Physiol Occup Physiol. 1993;66(6):542–546.
- Kolka MA, Stephenson LA. Exercise thermoregulation after prolonged wakefulness. J Appl Physiol. 1988;64(4):1575–1579.
- Moore JP, Harper Smith AD, Di Felice U, et al. Three nights of sleep deprivation does not alter thermal strain during exercise in the heat. Eur J Appl Physiol. 2013;113(9):2353–2360.
- Peever J, Fuller PM. The biology of REM sleep. Curr Biol. 2017;27(22):R1237–R1248.
- Kushida CA, Bergmann BM, Rechtschaffen A. Sleep deprivation in the rat: IV. Paradoxical sleep deprivation. Sleep. 1989;12(1):22–30.
- Bergmann BM, Everson CA, Kushida CA, et al. Sleep deprivation in the rat: V. Energy use and mediation. Sleep. 1989;12(1):31–41.
- Landis CA, Bergmann BM, Ismail MM, et al. Sleep deprivation in the rat: XV. Ambient temperature choice in paradoxical sleep-deprived rats. Sleep. 1992;15(1):13–20.
- Patchev V, Felszeghy K, Korányi L. Neuroendocrine and neurochemical consequences of long-term sleep deprivation in rats: similarities to some features of depression. Homeost Health Dis. 1991;33:97–108.
- Hoshino K. Food deprivation and hypothermia in desynchronized sleep-deprived rats. Braz J Med Biol Res. 1996;29:41–46.
- Jaiswal MK, Mallick BN. Prazosin modulates rapid eye movement sleep deprivation-induced changes in body temperature in rats. J Sleep Res. 2009;18(3):349–356.
- Vishwakarma LC, Sharma B, Singh V, et al. Acute sleep deprivation elevates brain and body temperature in rats. J Sleep Res. 2020. doi:10.1111/jsr.13030
- Fuller A, Carter RN, Mitchell D. Brain and abdominal temperatures at fatigue in rats exercising in the heat. J Appl Physiol. 1998;84(3):877–883.
- Racinais S, Cocking S, Périard JD. Sports and environmental temperature: from warming-up to heating-up. Temperature. 2017;4:227–257. doi:10.1080/23328940.2017.1356427.
- Galloway SD, Maughan RJ. Effects of ambient temperature on the capacity to perform prolonged cycle exercise in man. Med Sci Sports Exercise. 1997;29(9):1240–1249.
- Maia-Lima A, Ramos GP, Moraes MM, et al. Effects of precooling on 30-km cycling performance and pacing in hot and temperate environments. Int J Sports Med. 2017;38(1):48–54.
- Hasegawa H, Piacentini MF, Sarre S, et al. Influence of brain catecholamines on the development of fatigue in exercising rats in the heat. J Physiol. 2008;586(1):141–149.
- Drummond LR, Kunstetter AC, Vaz FF, et al. Brain temperature in spontaneously hypertensive rats during physical exercise in temperate and warm environments. PLoS One. 2016;11(5):e0155919.
- Pires W, Wanner SP, Lima MR, et al. Physical exercise performance in temperate and warm environments is decreased by an impaired arterial baroreflex. PLoS One. 2013;8:e72005.
- Müller-Ribeiro FC, Wanner SP, Santos WH, et al. Changes in systolic arterial pressure variability are associated with the decreased aerobic performance of rats subjected to physical exercise in the heat. J Therm Biol. 2017;63:31–40.
- Zheng X, Takatsu S, Ishikawa R, et al. Moderate intensity, exercise-induced catecholamine release in the preoptic area and anterior hypothalamus in rats is enhanced in a warm environment. J Therm Biol. 2018;71:123–127.
- Endo T, Schwierin B, Borbély AA, et al. Selective and total sleep deprivation: effect on the sleep EEG in the rat. Psychiatry Res. 1997;66(2–3):97–110.
- Damasceno WC, Pires W, Lima MR, et al. The dynamics of physical exercise-induced increases in thalamic and abdominal temperatures are modified by central cholinergic stimulation. Neurosci Lett. 2015;590:193–198.
- Wanner SP, Leite LH, Guimarães JB, et al. Increased brain l-arginine availability facilitates cutaneous heat loss induced by running exercise. Clin Exp Pharmacol Physiol. 2015;42(6):609–616.
- Suchecki D, Tufik S. Social stability attenuates the stress in the modified multiple platform method for paradoxical sleep deprivation in the rat. Physiol Behav. 2000;68(3):309–316.
- Machado RB, Hipólide DC, Benedito-Silva AA, et al. Sleep deprivation induced by the modified multiple platform technique: quantification of sleep loss and recovery. Brain Res. 2004;1004:45–51.
- Young AA, Dawson NJ. Evidence for on-off control of heat dissipation from the tail of the rat. Can J Physiol Pharmacol. 1982;60(3):392–398.
- Romanovsky AA. Skin temperature: its role in thermoregulation. Acta Physiol. 2014;210(3):498–507.
- Wanner SP, Guimarães JB, Rodrigues LO, et al. Muscarinic cholinoceptors in the ventromedial hypothalamic nucleus facilitate tail heat loss during physical exercise. Brain Res Bull. 2007;73(1–3):28–33.
- Malheiros-Lima MR, Pires W, Fonseca IAT, et al. Physical exercise-induced cardiovascular and thermoregulatory adjustments are impaired in rats subjected to cutaneous artery denervation. Front Physiol. 2018;9:74.
- Wanner SP, Prímola-Gomes TN, Pires W, et al. Thermoregulatory responses in exercising rats: methodological aspects and relevance to human physiology. Temperature. 2015;2(4):457–475. doi: 10.1080/23328940.2015.1119615.
- Cheuvront SN, Bearden SE, Kenefick RW, et al. A simple and valid method to determine thermoregulatory sweating threshold and sensitivity. J Appl Physiol. 2009;107(1):69–75.
- Hopkins WG, Marshall SW, Batterham AM, et al. Progressive statistics for studies in sports medicine and exercise science. Med Sci Sports Exerc. 2009;41(1):3–12.
- Tufik S, Troncone LR, Braz S, et al. Does REM sleep deprivation induce subsensitivity of presynaptic dopamine or postsynaptic acetylcholine receptors in the rat brain? Eur J Pharmacol. 1987;140(2):215–219.
- Cordeiro LMS, Rabelo PCR, Moraes MM, et al. Physical exercise-induced fatigue: the role of serotonergic and dopaminergic systems. Braz J Med Biol Res. 2017;50(12):e6432.
- Meeusen R, Roelands B. Central fatigue and neurotransmitters, can thermoregulation be manipulated?. Scand J Med Sci Sports. 2010;20:19–28.
- Mathes WF, Nehrenberg DL, Gordon R, et al. Dopaminergic dysregulation in mice selectively bred for excessive exercise or obesity. Behav Brain Res. 2010;210(2):155–163.
- Brock JW, Farooqui SM, Ross KD, et al. Stress-related behavior and central norepinephrine concentrations in the REM sleep-deprived rat. Physiol Behav. 1994;55(6):997–1003.
- Landis CA, Savage MV, Lentz MJ, et al. Sleep deprivation alters body temperature dynamics to mild cooling and heating not sweating threshold in women. Sleep. 1998;21(1):101–108.
- Endo T, Roth C, Landolt HP, et al. Selective REM sleep deprivation in humans: effects on sleep and sleep EEG. Am J Physiol. 1998;274:R1186–R1194.
- Müller-Ribeiro FC, Zaretsky DV, Zaretskaia MV, et al. Contribution of infralimbic cortex in the cardiovascular response to acute stress. Am J Physiol Regul Integr Comp Physiol. 2012;303(6):R639–R650.
- Pae YS, Lai H, Horita A. Hyperthermia in the rat from handling stress blocked by naltrexone injected into the preoptic-anterior hypothalamus. Pharmacol Biochem Behav. 1985;22(2):337–339.
- Kunstetter AC, Barbosa NHS, Moraes MM, et al. Pre-exercise exposure to the treadmill setup changes the cardiovascular and thermoregulatory responses induced by subsequent treadmill running in rats. Temperature. 2018;5(2018):109–122. doi: 10.1080/23328940.2017.1388343.
- Noakes TD. Fatigue is a brain-derived emotion that regulates the exercise behavior to ensure the protection of whole body homeostasis. Front Physiol. 2012;3:82.
- Kunstetter AC, Wanner SP, Madeira LG, et al. Association between the increase in brain temperature and physical performance at different exercise intensities and protocols in a temperate environment. Braz J Med Biol Res. 2014;47:679–688.
- Walters TJ, Ryan KL, Tate LM, et al. Exercise in the heat is limited by a critical internal temperature. J Appl Physiol. 2000;89(2):799–806.
- Machado FS, Rodovalho GV, Coimbra CC. The time of day differently influences fatigue and locomotor activity: is body temperature a key factor? Physiol Behav. 2015;140:8–14.
- Machado FS, Fóscolo DR, Poletini MO, et al. Influence of time-of-day on maximal exercise capacity is related to daily thermal balance but not to induced neuronal activity in rats. Front Physiol. 2016;7:464.
- Shellock FG, Rubin SA. Temperature regulation during treadmill exercise in the rat. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:1872–1877.
- Wilson NC, Gisolfi CV, Farber J, et al. Colonic and tail-skin temperature responses of the rat at selected running speeds. J Appl Physiol Respir Environ Exerc Physiol. 1978;44:571–575.
- Teixeira-Coelho F, Fonseca CG, Barbosa NHS, et al. Effects of manipulating the duration and intensity of aerobic training sessions on the physical performance of rats. PLoS One. 2017;12(8):e0183763.
- Cheung SS, Sleivert GG. Multiple triggers for hyperthermic fatigue and exhaustion. Exerc Sport Sci Rev. 2004;32(3):100–106.
- Cheuvront SN, Kenefick RW, Montain SJ, et al. Mechanisms of aerobic performance impairment with heat stress and dehydration. J Appl Physiol. 2010;109(6):1989–1995.
- Flouris AD, Schlader ZJ. Human behavioral thermoregulation during exercise in the heat. Scand J Med Sci Sports. 2015;25:52–64.
- Epstein Y, Roberts WO. The pathophysiology of heat stroke: an integrative view of the final common pathway. Scand J Med Sci Sports. 2011;21(6):742–748.
- Armstrong LE, Casa DJ, Millard-Stafford M, et al. American College of Sports Medicine position stand. Exertional heat illness during training and competition. Med Sci Sports Exercise. 2007;39:556–572.
- Hudson ASR, Soares ADN, Horta NAC, et al. The magnitude of physical exercise-induced hyperthermia is associated with changes in the intestinal permeability and expression of tight junction genes in rats. J Therm Biol. 2020;91:102610.
- Costa KA, Soares AD, Wanner SP. L-arginine supplementation prevents increases in intestinal permeability and bacterial translocation in male Swiss mice subjected to physical exercise under environmental heat stress. J Nutr. 2014;144:218–223.
- Bouchama A, Knochel JP. Heat stroke. N Engl J Med. 2002;346(25):1978–1988.
