 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Reflex cutaneous vasodilation during heating is attenuated in healthy human aging secondary to blunted increases in efferent skin sympathetic nervous system activity (SSNA) and reductions in end-organ sensitivity. Whether age-related alterations in the mean body temperature (b) threshold for increasing SSNA and/or the sensitivity of responses are evident with aging have not been examined. We tested the hypotheses that the Tb threshold for SSNA and cutaneous vascular conductance (CVC) would be increased, but the sensitivity would be reduced, with aging. Reflex vasodilation was induced in 13 young (23 ± 3 y) and 13 older (67 ± 7 y) adults using a water-perfused suit to systematically increase mean skin and esophageal temperatures. SSNA (peroneal microneurography) and red cell flux (laser Doppler flowmetry) in the innervated dermatome were continuously measured. SSNA was normalized to baseline; CVC was normalized as a percentage of maximal CVC. Baseline
b was lower in older adults (36.0 ± 0.4°C vs 36.4 ± 0.3°C; p = 0.005). During passive heating, the ∆
b thresholds for increasing SSNA and CVC were greater (1.3 ± 0.4°C vs 0.9 ± 0.3°C; p = 0.007 and 1.3 ± 0.4°C vs 0.8 ± 0.3°C; p = 0.002, respectively) in older adults. The slope of the relation between both SSNA (0.31 ± 0.23 vs 0.13 ± 0.10 V⋅s⋅°C −1; p = 0.01) and CVC (87.5 ± 50.1 vs 32.4 ± 18.1%max⋅°C−1; p = 0.002) vs
b was lower in older adults. The relative
b threshold for activation of SSNA and the initiation of reflex cutaneous vasodilation is higher in older adults, and once activated, the sensitivity of both responses is diminished, supporting the concept that the efferent component of the thermoregulatory reflex arc is impaired in healthy aging.
Abbreviations: CI: confidence interval; CVC: cutaneous vascular conductance; SSNA: skin sympathetic nervous system activity; b: mean body temperature; Tes: esophageal temperature;
sk: mean skin temperature.
Introduction
Climate change is the biggest global health threat of the 21st century and will continue to result in more intense, more frequent, and longer-lasting extreme heat events, all of which have dire implications for nearly every aspect of human life [Citation1]. The relation between heat waves and increased morbidity and mortality is well documented [Citation2–4]. Older adults, even those without underlying disease, are particularly vulnerable to intense and/or prolonged heat exposure [Citation5,Citation6]. Excessive heat-related mortality in aged adults can be attributed, at least in part, to the cardiovascular consequences of age-related impairments in thermoregulatory reflex function [Citation7,Citation8]. Given the rapidly aging global population [Citation9], it is imperative to more fully understand the mechanistic regulation of the integrated neural-cardiovascular response to heat exposure, with the ultimate goal of identifying possible intervention strategies to alleviate the increase in risk incurred by older adults.
In humans, the thermoregulatory system is governed by multiple interrelated independent neural reflex arcs [Citation10]. During whole-body heat exposure, an increase in skin and core tissue temperatures activates peripheral thermoreceptors. The resultant afferent neural signals are relayed to brainstem regions, primarily the preoptic anterior hypothalamus; central integration of this thermosensory input results in an increase in efferent skin sympathetic nervous system activity (SSNA) to activate eccrine sweating and cutaneous vasodilation – the primary thermoeffector mechanisms for heat dissipation [Citation10,Citation11]. A series of studies from our laboratory [Citation8,Citation11–13] has demonstrated significant functional deficits at multiple points along the efferent thermoregulatory reflex axis in healthy older adults. First, the increase in SSNA during whole-body heating is markedly blunted [Citation8,Citation12], a central neural limitation that appears to be specific to thermoregulatory stimuli [Citation8,Citation14]. Second, this attenuated efferent sympathetic response to heating is related to impaired reflex cutaneous vasodilation [Citation8,Citation12,Citation13] and, presumably, sweating [Citation15]. Further, sympathetic transduction to the cutaneous microvasculature during heat stress, estimated from the slope of the linear relation between increases in efferent SSNA and increases in microvascular cutaneous vascular conductance (CVC), is reduced in healthy older adults [Citation8,Citation12,Citation13], owing to reductions in both the sensitivity and the range of end-organ thermoeffector responsiveness. Finally, age-related impairments in both microvascular endothelial and sweat gland function during whole-body heat exposure are well established [Citation15–18] and contribute to compromised thermoregulatory function in older adults.
Considered collectively, this body of work demonstrates significant age-related functional impairments in the efferent arc of the thermoregulatory reflex axis. An additional interpretation of these data is that blunted efferent sympathetic outflow in response to heat exposure in older adults – and thus the ensuing impairments in thermoeffector function – is a consequence of alterations in either afferent signaling from peripheral thermoreceptors or the central integration of converging afferent signals, or both. Although these mechanistic possibilities are difficult, if not impossible, to directly and concurrently assess in humans, a recent study suggests that the temperature threshold at which heat-induced activation of SSNA first occurs, coupled with the overall gain of the response, may provide more specific insight for the neural control of thermoregulatory reflex function [Citation19].
Historically, descriptions of human thermoregulatory control have borrowed from systems engineering control theory, i.e. the control of continuously operating dynamical systems. While this analogy, often described as set-point theory, is not without its drawbacks, it does provide insights into thermoregulatory control characteristics [Citation20]. Briefly, an “error signal” deviation from a “set point” results in the initiation of, or increases in, efferent signaling. As the stimulus increases, this signal is turned on at a threshold proportional to the error signal. Once activated, the gain (or sensitivity of the response) is a proportional value that reflects the relation between the magnitude of the input signal (in this case, mean body temperature, b) and the magnitude of the output signal (SSNA and CVC/sweat rate). Retrospectively applying this control theory approach, we tested the hypothesis that the
b threshold for activation of SSNA would be increased, but, consistent with our previous studies [Citation8,Citation12,Citation13], the sensitivity of the response (gain) would be reduced, in healthy older compared to young adults.
Methods
All experimental procedures and protocols were approved by The Pennsylvania State University Institutional Review Board (protocols 1939 and 5791) and the Federal Drug Administration (FDA IND 103,180), and the study was conducted in accordance with the standards outlined in the Declaration of Helsinki. Informed verbal and written consent were obtained voluntarily from all volunteers prior to study participation. Data from a previously published study from our laboratory were retrospectively analyzed to test the novel hypotheses of the present investigation [Citation8].
Participants
All subjects underwent an initial health screening to determine study eligibility. This included a complete medical health history and physical examination, a resting 12-lead electrocardiogram, and 12-hour fasting blood biochemistry (Quest Diagnostics, Pittsburgh, PA). On a separate visit, participants (n = 10 per group) completed a graded treadmill exercise test (modified Bruce protocol) to determine VO2max (Parvo Medics, Salt Lake City, UT).
All participants were normotensive (resting seated systolic blood pressure <140 mmHg and diastolic blood pressure <90 mmHg), non-diabetic (HbA1c <5.7%), normocholesterolemic, non-obese (body mass index <30 kg/m2), normally active (neither sedentary nor endurance trained), and did not use tobacco products or over-the-counter or prescription medications or supplements with primary or secondary cardiovascular effects (e.g. statins, antihypertensives, anticoagulants, antidepressants, etc.). No participants had any evidence of overt cardiovascular disease or any evidence or diagnosis of associated comorbidities, including renal, pulmonary, metabolic, neurological, or dermatological disease (). Young women were tested during the early follicular phase of their menstrual cycle (n = 5) or during the placebo phase of hormonal contraceptive regimens (n = 1). Any older women currently or recently taking hormone therapy were excluded. All subjects were familiarized with the equipment and experimental protocol before testing.
Table 1. Participant characteristics
Experimental measurements
Copper-constantan thermocouples were affixed to the surface of the skin (calf, thigh, abdomen, chest, shoulder, and back) for the continuous unweighted measurement of mean skin temperature (sk). A copper-constantan thermocouple encased in the lumen of a sealed pediatric feeding tube was positioned in the esophagus at the level of the right atrium for the continuous measurement of esophageal temperature (Tes).
Heart rate was measured via a single-lead electrocardiogram (CardioCap, GE Healthcare, Milwaukee, WI). Respiratory excursions were monitored using a strain-gauge pneumography placed in a stable position over the abdomen (Pneumotrace, UFI, Morro Bay, CA). Blood pressure was obtained on a beat-to-beat basis using finger photoplethysmography (BMEYE, Nexfin, St. Louis, MO). Automated brachial artery blood pressure (CardioCap) was measured every 5 minutes throughout the experimental protocol and was used to verify absolute finger blood pressure measurements. To obtain an index of cutaneous blood flow, red blood cell flux was continuously measured directly on the dorsum of the foot, in the area of sympathetic innervation, using a laser Doppler flowmetry probe (moorLab, Moor Instruments, Axminster, UK). To specifically isolate reflex vasodilation, a local heating unit (Temperature Monitor, SHO2) holding the laser Doppler flowmeter was maintained at 33°C throughout whole-body heating. Because this is a retrospective analysis based on a series of studies that were originally designed to examine sympathetic neural control of cutaneous vasodilation during heating in healthy older adults [Citation8,Citation12,Citation13], measurements of sweat rate were not obtained.
Multiunit recordings of postganglionic SSNA were obtained by the insertion of a unipolar tungsten microelectrode (UNA35F2S, FHC Inc., Bowdoin, ME) percutaneously through the intact unanaesthetized skin and positioning into skin nerve fascicles of the peroneal nerve near the fibular head. A reference electrode (L-type, Seirin, Shizuoka, Japan) was inserted 2–3 cm from the recording microelectrode. The microelectrode was adjusted until bursts of SSNA occurred in response to light stroking of the dorsum of the foot [Citation21] and to arousal stimuli (loud noise) [Citation22]. Participants performed an end-expiratory apnea or a Valsalva maneuver to rule out any muscle sympathetic nerve activity [Citation23]. Neural signals were amplified, bandwidth filtered (700–2000 Hz), rectified, and integrated (time constant 0.1 sec) (Nerve Traffic Analyzer 662 C-4, University of Iowa Bioengineering, Iowa City, IA). Mean voltage neurograms were visually displayed and routed to a loudspeaker for continuous monitoring throughout the study. At the conclusion of the experiment, afferent and efferent SSNA responsiveness was elicited to confirm a consistent recording site.
Experimental protocol
Experimental visits were conducted in the morning. Prior to the experimental visit, participants abstained from eating for 4 hours, caffeine and alcohol for 12 hours, and strenuous physical activity for 24 hours. The protocol was conducted in a thermoneutral climate-controlled laboratory (22°C), and testing occurred with participants in the supine position.
Participants donned a tube-lined water-perfused suit that covered the entire body except for the head, hands, feet, and experimental leg and was used to control sk. After instrumentation and the obtainment of a suitable SSNA recording, baseline data were collected for 10 minutes at thermoneutrality. During this baseline period,
sk was maintained at ~34°C by perfusing 32°C water through the suit. Thereafter, 52°C water was perfused to increase and then clamp
sk at ~39°C and to gradually increase Tes by 1°C. Following whole-body heating, subjects were returned to thermoneutrality, and the temperature of the local heating unit was increased to 43°C to obtain a measurement of maximal cutaneous vasodilation for subsequent data normalization [Citation8,Citation12,Citation13].
Data and statistical analyses
All physiological signals were recorded at 40–1000 Hz (Powerlab 16/35, AD Instruments, Colorado Springs, CO). To account for the relative influence of core and skin temperatures throughout various phases of the protocol, different weighting coefficients were used for core and skin temperatures. Because a relatively greater proportion of b can be attributed to
sk in the absence of vasodilation [Citation24],
b was calculated as (0.67*Tes) + (0.33*
sk) during the thermoneutral baseline and the initial phase of whole-body heating prior to the threshold for CVC; thereafter,
b was calculated as (0.8*Tes) + (0.2*
sk). Data were also analyzed as a function of
b calculated as (0.9*Tes) + (0.1*
sk) and qualitatively similar results were obtained (data not shown). Consistent with previous studies, integrated SSNA was quantified as the area of all bursts within a region of interest, relative to a period of neural silence during thermoneutral baseline [Citation25]. Because the primary analyses in the present investigation do not directly compare SSNA responsiveness between groups, the increase in integrated SSNA is presented as an absolute value throughout heating. Cutaneous vascular conductance (CVC) was calculated as red blood cell flux (perfusion units) divided by mean arterial pressure and is expressed as a percentage of maximum [Citation8,Citation12,Citation13,Citation26,Citation27].
All data were averaged in 60 sec bins throughout the protocol. The last 60 sec of data prior to the onset of heating was used as the thermoneutral baseline. Two blinded investigators separately determined the temperatures (b, Tes, and
sk) at which SSNA and CVC first increased (onset threshold) and the temperatures at which SSNA and CVC plateaued by visual inspection () [Citation28]. The inter-rater reliability (ICC) was 0.92 for the onset threshold and 0.93 for the plateau, suggesting excellent reliability for this method. In the case of discrepancies >0.02°, the analysis was repeated. The slope of the linear portion of the relation between SSNA or CVC and temperature was then calculated (Prism8, GraphPad Software, San Diego, CA) and used as an index of the sensitivity of the response. In individuals in whom the onset threshold, and therefore slope, for SSNA could not be determined (young: n = 1 Tes; n = 2
sk; n = 1
b; older: n = 2 Tes; n = 4
sk; n = 2
b), data were excluded from analysis. Thresholds and slopes for CVC were obtained in all subjects in each group. Because group differences in
b at thermoneutrality were evident, data were analyzed for both absolute and relative increases in temperature during heating. Independent sample t-tests were used to evaluate group differences in onset threshold and sensitivity (SPSS, IBM, Armonk, NY). Pearson correlations were used to examine the relation between VO2max and the onset thresholds and slopes for both SSNA and CVC. Results are reported as means±standard deviation or 95% confidence interval, unless otherwise noted, and the ⍺-level was set at p < 0.05.
Figure 1. Representative data illustrating the profile of skin sympathetic nervous system activity (SSNA; Panel A) and cutaneous vascular conductance (CVC; Panel B) in response to whole-body passive heating-induced increases in mean body temperature (b) in one young (gray symbols) and one older adult (white symbols). The threshold was defined as the
b at which activation of SSNA and the initiation of reflex cutaneous vasodilation first occurred. The slope of the linear portion of the relation between
b and SSNA or CVC (demarcated by the threshold and the
b at which the response plateaued) was calculated by linear regression and used as an index of the thermosensitivity of the reflex response
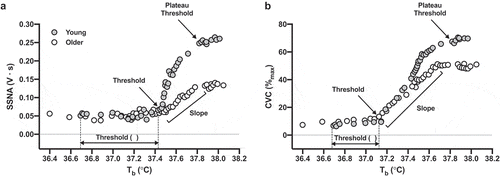
Results
At thermoneutrality, Tes was modestly lower in older adults (), though this did not reach statistical significance. However, both sk and
b during the thermoneutral baseline were reduced in older adults (). Neither baseline (young: 0.2 ± 0.1 vs. older: 0.1 ± 0.1 perfusion units/mmHg; p = 0.80) nor maximal CVC (young: 2.0 ± 0.5 vs. older: 1.7 ± 0.4 perfusion units/mmHg; p = 0.10) were different between groups. Absolute SSNA at rest was not different between groups (young: 1.0 ± 0.52 vs. older: 0.73 ± 0.59 volts; p = 0.23).
Table 2. Thermoneutral baseline and absolute onset threshold temperatures
Representative data depicting the b onset threshold and overall gain of the response (i.e. slope) for both SSNA and CVC are presented in . There were no group differences in the absolute
b thresholds for either SSNA or CVC (; all p > 0.05). However, because
b at baseline was lower in older adults, the increase in
b necessary to activate SSNA and elicit an increase in CVC was greater in older adults (). Greater increases in both Tes and
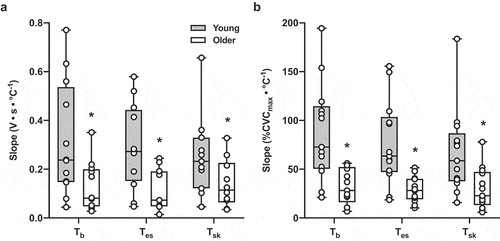
sk to initiate increases in SSNA and CVC were also evident in older adults (). Once activated, the sensitivity of the neural reflex response (i.e. the rate of increase in SSNA relative to heating-induced increases in temperature) was reduced in older adults (). The sensitivity of the cutaneous vasodilatory response was likewise blunted in older compared to young adults (). To confirm an adequate sample size to detect the noted group differences, post hoc power analyses were performed for the primary outcome variables. For the relative
b onset threshold for SSNA, the calculated effect size, after excluding those participants in whom this could not be determined (d = 1.19), yielded a power of 0.87, and for the sensitivity of the SSNA response, the calculated effect size (d = 1.09) yielded a power of 0.81.
Figure 2. The increase in mean body temperature (b; n = 12 young, n = 11 older), esophageal temperature (Tes; n = 11 young, n = 9 older), and mean skin temperature (
sk; n = 12 young, n = 11 older) required for activation of skin sympathetic nervous system activity (SSNA; Panel A) in young (gray bars) and older adults (white bars). In individuals in whom the threshold for SSNA could not be determined, data were excluded from analysis. The initiation of reflex cutaneous vasodilation is presented in Panel B (n = 13 young, n = 13 older). Mean summary data are presented as the median, first and third quartiles, and range (minimum and maximum), and group differences were analyzed by unpaired t-test. The
b onset threshold for both SSNA and cutaneous vascular conductance (CVC) was greater in older adults. *p < 0.05 vs. young
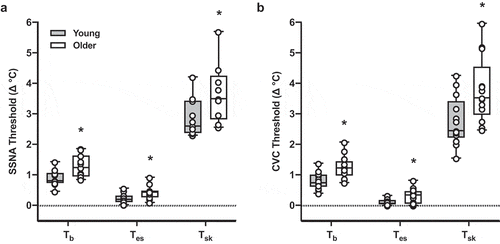
Figure 3. The slope of the linear portion of the relation between skin sympathetic nervous system activity (SSNA; Panel A) and cutaneous vascular conductance (CVC; Panel B) and increases in mean body temperature (b; n = 12 young, n = 11 older), esophageal temperature (Tes; n = 11 young, n = 9 older), and mean skin temperature (
sk; n = 12 young, n = 11 older) during passive heating in young (gray bars) and older adults (white bars). Mean summary data are presented as the median, first and third quartiles, and range (minimum and maximum), and group differences were analyzed by unpaired t-test. In individuals in whom the slope for SSNA could not be determined, data were excluded from analysis. The sensitivity of both SSNA and vasodilatory responsiveness was markedly reduced in older adults. *p < 0.05 vs. young
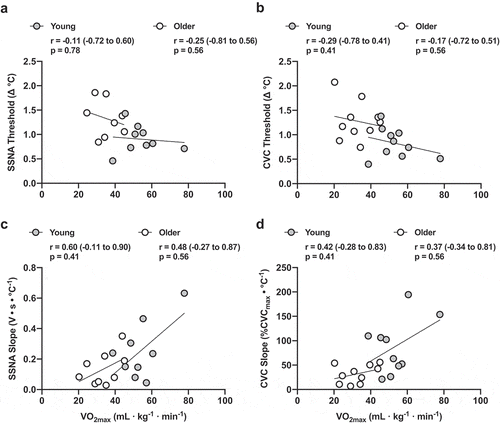
When including all participants, the relative increase in b necessary to elicit an increase in SSNA (r = −0.50 (95% CI: −0.79 to −0.02), p = 0.04) and initiate reflex cutaneous vasodilation (r = −0.54 (95% CI: −0.79 to −0.13), p = 0.01) was negatively related, and the slope (SSNA: r = 0.63 (95% CI: 0.23 to 0.85), p = 0.005; CVC: r = 0.64 (95% CI: 0.28 to 0.84), p = 0.002) was positively related, to VO2max, suggesting that maximal aerobic capacity may influence the neural control of thermoregulatory reflex function. However, when controlling for age, VO2max did not predict the relative
b onset threshold () or sensitivity of the response () for either SSNA or CVC.
Figure 4. The correlation (95% confidence interval) between maximal oxygen consumption (VO2max) and the relative mean body temperature (b) threshold for skin sympathetic nervous system activity (SSNA; n = 9 young, n = 8 older; Panel A) and reflex cutaneous vasodilation (n = 10 young, n = 10 older; Panel B) during passive heating in young (gray symbols) and older adults (white symbols). The relation between VO2max and the sensitivity of SSNA and vasodilatory responsiveness are presented in Panels C and D
Discussion
The primary novel finding of this retrospective analysis was that the increase in b necessary to elicit activation of SSNA and the subsequent initiation of reflex cutaneous vasodilation was greater in healthy older adults than in their young counterparts. Further, consistent with a series of recent studies from our laboratory [Citation8,Citation12,Citation13], the analytical approach employed in the present investigation also indicated a significant age-related reduction in the sensitivity of the efferent reflex (SSNA) arc and thermoeffector (CVC) responsiveness during passive heat exposure. Within each age cohort, aerobic fitness did not predict the relative
b onset threshold or sensitivity for either SSNA or reflex vasodilation. Taken together, these data confirm and extend our understanding of impaired neural control of thermoregulatory reflex function in healthy aging, even in the absence of overt neurocardiovascular disease.
Healthy human aging is associated with impaired thermoregulation, historically attributed in large part to impairments in the thermoeffector mechanisms for heat dissipation – sweating and cutaneous vasodilation [Citation15–18]. The efferent component of the neural reflex arc that controls activation of eccrine sweating and cutaneous vasodilation during whole-body heat exposure originates with large increases in SSNA [Citation8,Citation19,Citation29–31]. In a recent series of studies, we systematically examined sympathetic control of reflex cutaneous vasodilation during passive heating in healthy older adults [Citation8,Citation12,Citation13]. We reported two distinct age-related deficits in efferent neural function: 1) attenuated increases in SSNA throughout passive heating and 2) blunted transduction of SSNA to cutaneous vasodilatory responsiveness [Citation8]. In order to provide data that were directly comparable to previous studies conducted by our laboratory over the past two decades [Citation8,Citation11–13,Citation15,Citation16,Citation32], our recent investigations of efferent SSNA during heat exposure utilized water-perfused suit-induced increases in skin temperature to drive, and subsequently clamp, an increase in core temperature of 1.0°C [Citation8,Citation12,Citation13]. As such, data were analyzed at progressive increments in core temperature throughout heating, making comparisons between studies more valid. However, in this experimental approach of passive heating, there is a window of time in which skin temperature is rising rapidly (the initial ~10 minutes), while core temperature remains the same. Thus, indexing SSNA (and CVC) relative to increases in b, as well as to Tes and
sk, to account for, and separately examine, the influence of both core and skin temperatures may provide additional novel insight into potential age-related differences in the neural control of thermoregulation.
The rationale underlying the complementary analytical approach used in this investigation is built upon the seminal works of Stolwijk and Hardy [Citation20], as well as Nadel et al. [Citation28,Citation33], who first described the model of thermoregulatory function whereby the b at which thermoeffector output begins to increase (i.e. threshold) reflects “central” control, whereas the slope of the relation between
b and thermoeffector output (i.e. sensitivity) reflects “peripheral” control. This interpretation has been both supported and refuted in the intervening years [Citation34–37]. However, within this conceptual framework, central control of thermoregulation encompasses afferent signaling from thermoreceptors, neural integration of thermosensory input, and efferent outflow to thermoeffectors; peripheral control of thermoregulation comprises the end-organ thermoeffector responses of sweating and cutaneous vasodilation [Citation38].
Using this analytical approach, the present data indicate that the increase in b necessary to elicit an increase in SSNA during heat exposure was greater in older adults, likely owing to the observed reductions in basal core and skin temperatures. Older adults also demonstrated a greater relative threshold for eliciting reflex cutaneous vasodilation. Experimentally, the use of a water-perfused suit to increase
b prevents effective heat dissipation; however, presumably the delayed activation of cutaneous vasodilation, coupled with reduced microvascular sensitivity to sympathetic outflow [Citation8,Citation12] and marked microvascular endothelial dysfunction [Citation11,Citation16,Citation32], contributes to the inability of older adults to appropriately modulate body temperature. A recent study employed this experimental and analytical approach to examine the effect of acute heat therapy on the neural control of body temperature in young adults [Citation19]. To account for the heat therapy-induced reductions in
b and absolute onset thresholds, both hallmark characteristics of heat acclimation [Citation28,Citation39,Citation40], these authors likewise relied upon the relative increase in
b required to elicit increases in SSNA as their primary outcome variable [Citation19]. The relative onset threshold for SSNA, as well as for sweat rate and skin blood flow, was reduced following acute heat therapy [Citation19], which was interpreted to reflect an improvement in the efferent neural thermoeffector reflex arc. Interestingly, prior to heat therapy, the relative
b onset threshold for activation of SSNA was ~1.0°C [Citation19], which is consistent with the data in young adults in the present study (see ), thus providing additional support for our interpretation that the relative
b onset threshold for activation of SSNA is greater in healthy older adults.
In the present study, older adults also exhibited marked reductions in the sensitivity of both SSNA and vasodilatory responsiveness to passive heating. That is, the rate of the increase in sympathetic outflow and skin blood flow throughout progressive increases in b was lower in older adults, and this likely contributes, at least in part, to the age-related reduction in the overall magnitude of the increase in SSNA and CVC in response to heat exposure [Citation8,Citation12,Citation13,Citation16,Citation32]. However, a limitation of the present analytical approach is that it only provides information on efferent outflow and thermoeffector output, and the functional link between these two components of the reflex arc – that is, the transduction of the neural signal into a sudomotor and vasomotor response – was not addressed. We previously demonstrated a reduction in both the range and slope of the linear relation between SSNA and CVC during heating in older adults [Citation8], which is arguably a more relevant approach for examining the entire efferent thermoregulatory reflex axis. Nevertheless, the current data, coupled with our earlier findings [Citation8,Citation12,Citation13], provide compelling support for the conclusion that blunted increases in efferent SSNA directly contribute to marked impairments in reflex cutaneous vasodilation in healthy older adults.
Because efferent outflow is downstream of both afferent signaling and central neural integration, we posit that one or the other, or both, are also impaired in aging, thereby mediating the noted impairments in the efferent reflex arc in healthy aging. Because the ability to sense changes in skin temperature is the primary trigger for behavioral adaptations to heat exposure [Citation41,Citation42], the evidence suggesting that thermal perception decreases with age [Citation43] provides indirect support for the concept that afferent thermosensory function may be impaired in older adults. In line with this, in the present study, older adults demonstrated a greater relative sk onset threshold for SSNA, which may also suggest reduced afferent thermosensory function. Functional deficits in the processing and integration of afferent thermosensory inputs within the brain itself are certainly also plausible, particularly given the abundant evidence that a decline in sensory processing is characteristic of the aging process [Citation44]. However, to our knowledge, neither of these mechanistic possibilities have been directly tested in older adults and therefore are an exciting avenue of future inquiry.
In addition to providing a conceptual model of thermoregulation, the foundational studies by Nadel et al. [Citation28,Citation33] also examined the influence of aerobic exercise training and heat acclimation on central and peripheral control of thermoeffector function. Exercise training increased the slope of the relation between Tb and sweat rate but did not affect the onset threshold, whereas heat acclimation reduced the onset threshold for sweating without affecting the sensitivity of the b:sweat rate relation [Citation28]. That is, exercise training-induced improvements in thermoeffector function occur through peripheral mechanisms and those following heat acclimation occur through central mechanisms. Although the influence of aerobic fitness on the neural control of thermoregulatory reflex function was not a primary outcome of this study, given the noted influence of exercise training on peripheral thermoeffector function, we performed ancillary analyses exploring the relation between VO2max, a proxy for aerobic fitness, and the onset thresholds and sensitivities for both SSNA and CVC. When performing these analyses in the full sample of participants without regard for age, the relative onset thresholds and slopes for SSNA and CVC were linearly related to maximal oxygen consumption such that the onset threshold was lower, and the slope was steeper, in those adults who were more fit. These data suggest that aerobic capacity improves the efferent thermoregulatory reflex arc. However, when controlling for age, the predictive strength of VO2max was no longer apparent, likely owing to the small sample size within each age group for these types of statistical analyses. It is important to note that although VO2max was consistently lower in older adults, because this was not an a priori component of our study design, the range of VO2max values within each group was relatively narrow. In addition, habitual physical activity was not quantified. To more specifically examine the influence of fitness on these parameters in older adults, future studies should include both sedentary and exercise-trained men and women.
Although there were statistically significant differences in body mass and BMI between groups, these differences were likely negligible in terms of their potential to influence the rate of heat absorption. Even a 20% difference in fat mass causes only a minor difference (∼3–5%) in mean specific heat of the total body [Citation45]. Therefore, body morphology likely played no role in the age-related differences in the relative onset thresholds for activation of SSNA and reflex vasodilation.
In conclusion, the results presented herein demonstrate that the relative b threshold for the activation of SSNA and the initiation of reflex cutaneous vasodilation during passive heating is greater in healthy older adults. These data also indicate a reduction in the sensitivity of the efferent reflex arc and thermoeffector responsiveness during passive heat exposure in older adults, a finding that is consistent with previous studies from our laboratory in which we employed a more traditional analytical approach [Citation8,Citation12,Citation13]. Although the present study design precludes specific conclusions related to the maintenance of thermal homeostasis, speculatively, the delayed activation and reduced sensitivity of peripheral thermoeffector mechanisms likely have significant functional consequences for heat dissipation and the modulation of body temperature during heat exposure in older adults.
Key points summary
Reflex cutaneous vasodilation during heating is attenuated in healthy human aging secondary to blunted increases in efferent skin sympathetic nervous system activity (SSNA) and reductions in end-organ sensitivity.
The present findings demonstrate that the relative
b threshold for activation of SSNA and the initiation of reflex vasodilation is higher in older adults, and once activated, the sensitivity of both responses is diminished, supporting the concept that the efferent component of the thermoregulatory reflex arc is impaired in healthy aging.
The delayed activation and reduced sensitivity of peripheral thermoeffector mechanisms likely have significant functional consequences for heat dissipation and the modulation of body temperature during heat exposure in older adults.
Author contributions
JLG: conceived and designed research, data collection, analysis, interpretation, and manuscript preparation; AES: conceived and designed research, data collection, analysis, interpretation, and manuscript preparation; STW: data analysis, interpretation, and manuscript preparation; WLK: conceived and designed research, data analysis, interpretation, and manuscript preparation. All authors approved the final version of the manuscript. All laboratory work was conducted at PSU.
Acknowledgments
We greatly appreciate the effort expended by the volunteer participants. We thank Susan Slimak, RN and Jane Pierzga, MS for their assistance.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Watts N, Amann M, Arnell N, et al. The 2018 report of the Lancet Countdown on health and climate change: shaping the health of nations for centuries to come. Lancet. 2018;392:2479–2514.
- Azhar GS, Mavalankar D, Nori-Sarma A, et al. Heat-related mortality in India: excess all-cause mortality associated with the 2010 Ahmedabad heat wave. PLoS One. 2014;9:e91831.
- Fouillet A, Rey G, Laurent F, et al. Excess mortality related to the August 2003 heat wave in France. Int Arch Occup Environ Health. 2006;80:16–24.
- Whitman S, Good G, Donoghue ER, et al. Mortality in Chicago attributed to the July 1995 heat wave. Am J Public Health. 1997;87:1515–1518.
- Semenza JC, Rubin CH, Falter KH, et al. Heat-related deaths during the July 1995 heat wave in Chicago. N Engl J Med. 1996;335:84–90.
- Vandentorren S, Bretin P, Zeghnoun A, et al. August 2003 heat wave in France: risk factors for death of elderly people living at home. Eur J Public Health. 2006;16:583–591.
- Minson CT, Wladkowski SL, Cardell AF, et al. Age alters the cardiovascular response to direct passive heating. J Appl Physiol (1985). 1998;84:1323–1332.
- Stanhewicz AE, Greaney JL, Alexander LM, et al. Blunted increases in skin sympathetic nerve activity are related to attenuated reflex vasodilation in aged human skin. J Appl Physiol (1985). 2016;121:1354–1362.
- Harper S. Economic and social implications of aging societies. Science. 2014;346:587–591.
- Romanovsky AA. The thermoregulation system and how it works. Handb Clin Neurol. 2018;156:3–43.
- Holowatz LA, Kenney WL. Peripheral mechanisms of thermoregulatory control of skin blood flow in aged humans. J Appl Physiol (1985). 2010;109:1538–1544.
- Greaney JL, Stanhewicz AE, Kenney WL. Chronic statin therapy is associated with enhanced cutaneous vascular responsiveness to sympathetic outflow during passive heat stress. J Physiol. 2019;597:4743–4755.
- Stanhewicz AE, Greaney JL, Alexander LM, et al. Folic acid supplementation increases cutaneous vasodilator sensitivity to sympathetic nerve activity in older adults. Am J Physiol Regul Integr Comp Physiol. 2017;312:R681–R688.
- Greaney JL, Stanhewicz AE, Kenney WL, et al. Impaired increases in skin sympathetic nerve activity contribute to age-related decrements in reflex cutaneous vasoconstriction. J Physiol. 2015;593:2199–2211.
- Smith CJ, Alexander LM, Kenney WL. Nonuniform, age-related decrements in regional sweating and skin blood flow. Am J Physiol Regul Integr Comp Physiol. 2013;305:R877–885.
- Holowatz LA, Houghton BL, Wong BJ, et al. Nitric oxide and attenuated reflex cutaneous vasodilation in aged skin. Am J Physiol Heart Circ Physiol. 2003;284:H1662–1667.
- Inoue Y, Havenith G, Kenney WL, et al. Exercise- and methylcholine-induced sweating responses in older and younger men: effect of heat acclimation and aerobic fitness. Int J Biometeorol. 1999;42:210–216.
- Kenney WL. Control of heat-induced cutaneous vasodilatation in relation to age. Eur J Appl Physiol Occup Physiol. 1988;57:120–125.
- Barry H, Chaseling GK, Moreault S, et al. Improved neural control of body temperature following heat acclimation in humans. J Physiol. 2020;598:1223–1234.
- Stolwijk JA, Hardy JD. Temperature regulation in man–a theoretical study. Pflugers Arch Gesamte Physiol Menschen Tiere. 1966;291:129–162.
- Lawrence SJ, Botte MJ. The deep peroneal nerve in the foot and ankle: an anatomic study. Foot Ankle Int. 1995;16:724–728.
- Vallbo AB, Hagbarth KE, Torebjork HE, et al. Somatosensory, proprioceptive, and sympathetic activity in human peripheral nerves. Physiol Rev. 1979;59:919–957.
- Delius W, Hagbarth KE, Hongell A, et al. Manoeuvres affecting sympathetic outflow in human skin nerves. Acta Physiol Scand. 1972;84:177–186.
- Brengelmann GL, Wyss C, Rowell LB. Control of forearm skin blood flow during periods of steadily increasing skin temperature. J Appl Physiol. 1973;35:77–84.
- Greaney JL, Kenney WL. Measuring and quantifying skin sympathetic nervous system activity in humans. J Neurophysiol. 2017;118:2181–2193.
- Kirkman DL, Ramick MG, Muth BJ, et al. Effects of aerobic exercise on vascular function in nondialysis chronic kidney disease: a randomized controlled trial. Am J Physiol Renal Physiol. 2019;316:F898–F905.
- Wardell K, Braverman IM, Silverman DG, et al. Spatial heterogeneity in normal skin perfusion recorded with laser Doppler imaging and flowmetry. Microvasc Res. 1994;48:26–38.
- Nadel ER, Pandolf KB, Roberts MF, et al. Mechanisms of thermal acclimation to exercise and heat. J Appl Physiol. 1974;37:515–520.
- Cui J, Sathishkumar M, Wilson TE, et al. Spectral characteristics of skin sympathetic nerve activity in heat-stressed humans. Am J Physiol Heart Circ Physiol. 2006;290:H1601–1609.
- Gagnon D, Romero SA, Ngo H, et al. Plasma hyperosmolality attenuates skin sympathetic nerve activity during passive heat stress in humans. J Physiol. 2016;594:497–506.
- Wilson TE, Cui J, Crandall CG. Absence of arterial baroreflex modulation of skin sympathetic activity and sweat rate during whole-body heating in humans. J Physiol. 2001;536:615–623.
- Stanhewicz AE, Alexander LM, Kenney WL. Oral sapropterin acutely augments reflex vasodilation in aged human skin through nitric oxide-dependent mechanisms. J Appl Physiol (1985). 2013;115:972–978.
- Nadel ER, Mitchell JW, Saltin B, et al. Peripheral modifications to the central drive for sweating. J Appl Physiol. 1971;31:828–833.
- Henane R, Flandrois R, Charbonnier JP. Increase in sweating sensitivity by endurance conditioning in man. J Appl Physiol Respir Environ Exerc Physiol. 1977;43:822–828.
- Kellogg DL Jr., Pergola PE, Piest KL, et al. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res. 1995;77:1222–1228.
- Shibasaki M, Crandall CG. Effect of local acetylcholinesterase inhibition on sweat rate in humans. J Appl Physiol (1985). 2001;90:757–762.
- Tankersley CG, Smolander J, Kenney WL, et al. Sweating and skin blood flow during exercise: effects of age and maximal oxygen uptake. J Appl Physiol (1985). 1991;71:236–242.
- Periard JD, Travers GJS, Racinais S, et al. Cardiovascular adaptations supporting human exercise-heat acclimation. Auton Neurosci. 2016;196:52–62.
- Adolph EF. General and specific characteristics of physiological adaptations. Am J Physiol. 1956;184:18–28.
- Nielsen B, Hales JR, Strange S, et al. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. J Physiol. 1993;460:467–485.
- Schlader ZJ, Simmons SE, Stannard SR, et al. The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiol Behav. 2011;103:217–224.
- Schlader ZJ, Vargas NT. Regulation of body temperature by autonomic and behavioral thermoeffectors. Exerc Sport Sci Rev. 2019;47:116–126.
- Taylor NA, Allsopp NK, Parkes DG. Preferred room temperature of young vs aged males: the influence of thermal sensation, thermal comfort, and affect. J Gerontol A Biol Sci Med Sci. 1995;50:M216–221.
- Rossini PM, Rossi S, Babiloni C, et al. Clinical neurophysiology of aging brain: from normal aging to neurodegeneration. Prog Neurobiol. 2007;83:375–400.
- Geddes LA, Baker LE. The specific resistance of biological material–a compendium of data for the biomedical engineer and physiologist. Med Biol Eng. 1967;5:271–293.
