ABSTRACT
Enhanced intestinal permeability is a pervasive issue in modern medicine, with implications demonstrably associated with significant health consequences such as sepsis, multiorgan failure, and death. Key issues involve the trigger mechanisms that could compromise intestinal integrity and increase local permeability allowing the passage of larger, potentially dangerous molecules. Heat stress, whether exertional or environmental, may modulate intestinal permeability and begs interesting questions in the context of global climate change, increasing population vulnerabilities, and public health. Emerging evidence indicates that intestinal leakage of digestive enzymes and associated cell dysfunctions––a process referred to as autodigestion––may play a critical role in systemic physiological damage within the body. This increased permeability is exacerbated in the presence of elevated core temperatures. We employed Latent Dirichlet Allocation (LDA) topic modeling methods to analyze the relationship between heat stress and the nascent theory of autodigestion in a systematic, quantifiable, and unbiased manner. From a corpus of 11,233 scientific articles across four relevant scientific journals (Gut, Shock, Temperature, Gastroenterology), it was found that over 1,000 documents expressed a relationship between intestine, enhanced permeability, core temperature, and heat stress. The association has grown stronger in recent years, as heat stress and potential autodigestion are investigated in tandem, yet still by a limited number of specific research studies. Such findings justify the design of future studies to critically test novel interventions against digestive enzymes permeating the intestinal tract, especially the small intestine.
Introduction
Heat stress and associated conditions that may elevate core body temperature have received increasing attention in recent research and media reports due to global trends in climate change and urban growth, together causing increased heat exposures [Citation1]. Overexposure to extreme heat is often associated with discomfort, body temperature increases, and enhanced fluid losses (heat stress), the ensuing physiological responses, such as cardiovascular strain, dehydration, the body reaching severe levels of inflammation, cellular dysfunction, and organ failure, are referred to as heat strain [Citation2]. However, there is minimal agreement concerning the exact mechanisms that lead to circulatory and organ dysfunction after heat stress. A literature search by keywords in a medical database, such as “Heat Stress,” “Multi-Organ Failure,” or “Systemic Inflammatory Response Syndrome” (SIRS), yields tens of thousands of publications. If the search includes other keywords, ranging from dehydration [Citation3–7] to intestinal permeability, cytokines, and endotoxins [Citation8–29], the bodies of evidence become even more immense and rife with topical overlap.
In this comprehensive summary, we focus on mechanisms of organ failure during and after heat exposure. Specifically, we focus on the intestine and a recently identified pathogenic mechanism involving digestive enzymes, referred to as “autodigestion” [Citation28]. Autodigestion (AD) is the digestion of tissue in the body by digestive enzymes. These enzymes are synthesized in the pancreas and discharged into the small intestine for food digestion. Digestive enzymes require compartmentalization in the intestinal tract, which is provided by the mucosal epithelial barrier. However, digestive enzymes can leak into the central circulation should the intestine’s barrier become compromised. Although there may be several effectors in this process, instances of intestinal barrier dysfunction have been linked to high core temperatures [Citation29–44]. In systemic circulation, digestive enzymes generate a pathogenic process that leads to multiorgan dysfunction and/or failure. Given the novelty of the autodigestion theory, its relationship with heat exposure remains to be directly examined. Further, heat stress and autodigestion share co-morbidities, such as septic shock and multiorgan failure. Here we explore implicit and explicit connections between these occurrences, as well as the risk of an elevated body temperature partaking in the breakdown of the intestinal barrier for digestive enzymes.
These ideas are presented herein with two approaches: (1) a literature search aided by machine learning algorithms due to the large size of the literature on heat stress, strain, and morbidities; (2) a comprehensive summary and discussion of the hypothesis using a narrower subset of literature that examines a possible involvement of autodigestion in heat mortality and morbidity. The discussion is intended to stimulate future studies to systematically examine critical mechanisms that can lead to short- or long-term complications upon exposure to heat, and even lethal outcomes due to multiorgan failure. It should be noted that the intestine normally exhibits permeability to smaller molecules, which is essential to allow nutrients (amino acids, monosaccharides, etc.) to pass through the gut and is thus a normal physiological process. Moreover, intestinal permeability also rises under basal conditions and may be crucial for priming the intestinal immune system. In this summary, we focus on a more enhanced, or elevated, intestinal permeability that would allow larger molecules and even bacteria to pass and may be caused by higher core temperatures.
An unbiased machine-based literature review
Overview
The relationship between heat stress and autodigestion theory has not been explicitly investigated, but the literature is rich with implicit topical overlap. To comprehensively examine a large body (“corpus”) of literature and explore potential relationships between heat stress and intestinal co-morbidities of autodigestion, topic modeling (machine learning) methods are useful tools. This review employed a probabilistic topic modeling algorithm––Latent Dirichlet Allocation (LDA) [Citation45] to help overcome the disparate volumes of implicit and explicit evidence regarding this emerging theory and connections. This resampling algorithm describes a document as a relative ratiometric culmination of topics (e.g., heat, temperature, intestine, enzyme), where each topic is a subsequent culmination of certain words or segments of a few words called n-grams. This method avoids biases that may arise from selected and subjective decisions for articles used in the reviews from a large and unfocused corpus of literature, and further identifies and agglomerates underlying topics in an unbiased manner [Citation45]. Even if the autodigestion theory were more established, topics are not always detected via keyword searches from traditional literature reviews [Citation46]. Moreover, keywords do not necessarily span the entirety of a publication, and additional information that is not reflected by the keywords may contribute to understanding topical intersections and the pervasiveness of a conceptual consensus [Citation46]. Implicit discussion of topics and other phenomena may also stimulate and guide research. For example, articles that employ or mention an experimental technique bolster the efficacy and adoption of that technique, even if it is not the article’s focus. Similarly, discussion of biological concepts and theories lends to the richness and breadth of adoption in the scientific climate of those subjects, even if they are not the focus of the overall discussion. For further description and rationale of the LDA, see Supplement Material A.
Methodological processes: techniques, classifiers, and network analysis
In total, 11,233 scientific articles published over the last decade (2009–2021) were selected from journals that encompass the intestine and heat, specifically Gut, Shock, Temperature, and Gastroenterology. Articles were downloaded from the journal website or through a citation manager (Endnote; Clarivate Analytics). Letters to the editor, status or operational announcements, and in-memoriam dedications were mostly omitted. Articles were preprocessed by removing type-case, common words with little semantic meaning, and various punctuation. Retained words were stemmed to ignore plurality and other aspects of the same word. The resultant information is summarized in . Although these journals were already relevant to the topics of heat stress, intestine, and autodigestion, a smaller corpus was selected through a whole-text term-frequency inverse document-frequency (TF-IDF) screener [Citation47]. This screener, which retained the top 10% of documents related to heat stress and autodigestion co-morbidities, served to reduce computation time and improve the quality of the uncovered topics while retaining a large body of publications that would otherwise be impractical to manually read systematically. TF-IDF is a technique to retrieve relevant information for a query by considering both the occurrence and relative significance of terms in both the query and retrieved document.
Table 1. Corpus breakdown with basic information of documents in the selected journalsa
The retained documents were then subject to an Error Correcting Output Codes (ECOC) classifier [Citation48] – – a multiclass binary model for support vector machines (SVM) – – where they were classified as either heat illness related or intestine/autodigestion related, after being trained with terms described in . Since the documents retained by the TF-IDF step originated from the top 10% of relevant journal articles, those with posteriors closest to 50% are more likely to express greater topical overlap between heat stress and autodigestion than neither topic at all. However, the majority of the documents were still concentrated at the poles, which suggests that the documents of interest infrequently discuss heat stress and autodigestion tandemly and to similar extents (see also ).
Figure 1. ECOC Classification Training (a) Verification that highly relevant articles to either heat stress or autodigestion phenomena seldom discuss both topics in tandem and to similar extents
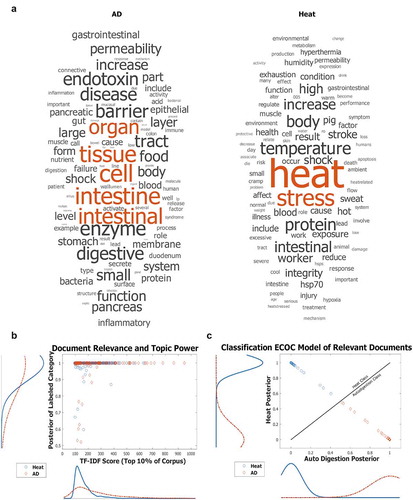
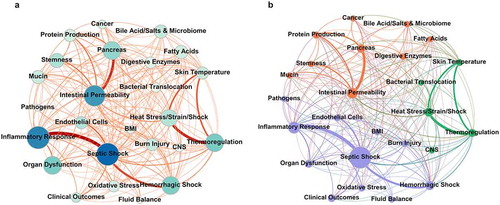
Documents were progressively split so that the distribution of document length was less skewed. LDA was performed on the processed corpus to generate topic probabilities and clarify the research climate, including trends over the last decade. Topic probabilities in split documents were then recombined such that they reflect the article from which they were derived. That is, if a longer document thoroughly discussed two topics in separate sections and was split roughly in the middle, the topic probabilities would appear to be a single document discussing both topics, as opposed to two documents discussing just one topic exclusively. A network diagram () was generated to display the topical relationships within the corpus. Topics are represented as nodes in the network, and when a document discusses multiple topics, these nodes are connected by an edge (line). Concurrently discussed topics have a thicker edge if the relationship occurs more frequently in the corpus.
Figure 2. Network maps of LDA Relationships display the topic distributions uncovered from the top 10% of TF-IDF filtered publications. Short-form topic names were manually assigned to LDA-clustered words that represent topics to allow for easier visualization. Edges that connect topic nodes are scaled in size and color by their tandem occurrence in a document. Each document consists of various proportions of all topics. Those topics that account for less than 1/nTopics in an article are removed from consideration for that article and the remaining articles' percentages of accountancy for that document are reweighted. (a) Network map with each node representing a topic. Node size is determined by closeness to centrality. Node color is scaled by betweenness centrality, with darker nodes representing nodes that connect the most topics in any given article within the top 10% percent of TF-IDF filtered papers. Edges connecting two nodes are scaled in color gradient and size by how often the two topics appear in the same report paper. (b) The network map with randomized modularity class detection with a modularity of 0.304 and a resolution of 1. Size of nodes is determined by how often that topic appears in articles of the top 10% percent of TF-IDF filtered papers. Three categories of topics (shown in purple, green, and orange) were uncovered by the network alone, without human intervention or semantic supervision. TF-IDF – Term Frequency Inverse Document Frequency, LDA – Latent Dirichlet Allocation, CNS – Central Nervous System, BMI – Body Mass Index
Interpretation & discussion of results
The resultant network diagram has a three-fold function:
It is a proposal for a novel, systematic, and unbiased method of reviewing the literature.
It aids in the understanding and visualization of relationships and trends in large bodies of research; and
It reinforces the validity of the topical relationship consensus drawn from the traditional literature review.
The network diagram outlines the topical expression of the analyzed corpus (). shows that co-morbidities of “shock” and “inflammatory response” serve as the most common bridge between topics and groups of topics, as shown by the darker nodes having a higher betweenness centrality score. Node size is scaled by closeness centrality, indicating that although certain topics may be highly connected, they are not necessarily the ones that are most frequently found in the corpus. illustrates three modularity classes uncovered by the network analysis. Despite screening just two major topics (heat stress and autodigestion), they do not frequently appear in the same text without the context of co-morbidities.
The benefits of network diagram representation include readily apparent measures of centrality. Nodes with high closeness centrality represent topics that may be associated with many other topics. Nodes with high betweenness centrality may not have so many edges yet may show important links between groups of topics. For instance, word-level topics of “septic shock,” “heat stress/strain/shock,” and “intestinal permeability” may be related to a similar number of other topics, but it is the topic of “septic shock” that acts as a liaison between groups of topics. This example indicates that the literature may associate “intestinal permeability” related topics with “heat stress/strain/shock” related topics less frequently than it associates each of them with “septic shock.” This finding indicates a transitive type of connection between heat illness and autodigestion related topics by means of co-morbidities. Additionally, both topic frequency and topic relationship frequency are readily apparent through the size of the nodes and edges. Modularity classes can also be highlighted to help readers visualize community detection and reinforce the understanding that topics may be hierarchical, whereby specific topics can be grouped into broader subjects, fields of study, or phenomenological events. As seen from , the word-level topics of “septic shock” and “inflammatory response” can be grouped in a broader category of morbidities, just like topics of “intestinal permeability” and “heat illness” also belong to distinct broader categories. (Supplemental Material Figure S.1 illustrates these measures in a simplified manner).
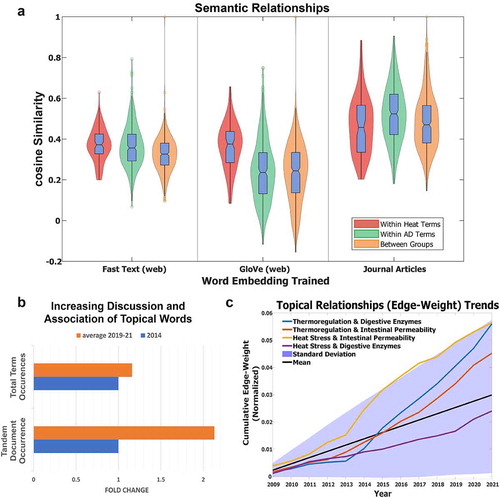
Furthermore, Latent Semantic Analysis (LSA) was conducted on the top 1% of words within heat illness or autodigestion related topics. This process led to slightly improved semantic relationships between autodigestion and heat illness related words (). Top n-grams from each of the three modularity classes uncovered in and their occurrences over time are depicted in . Years 2014 and 2019–2021 were selected because the journal Temperature had not been established prior. In recent years, the total term occurrences for both “Heat” and “AD” have increased. Additionally, the number of documents that mention them tandemly and in relation to co-morbidities, such as SIRS, shock, sepsis, and organ dysfunction have increased sharply. Not only are the raw term occurrences increasing, but their topical relationships are as well (). In fact, the rate at which topics of “heat stress” and “thermoregulation” connect with “intestinal permeability” and “digestive enzymes” are among the fastest-growing relationships in the network, despite relatively stable topical expression (see Supplemental Material B, Figure S.2 for this stability). This finding demonstrates that although the topic prevalence has remained relatively consistent, they are increasingly related to each other (Supplemental Material B, Figure S.3). A deeper understanding of the relationships among these topics will aid in understanding individual-level vulnerabilities that affect one’s susceptibility to heat stroke and death.
Figure 3. (a) Latent Semantic Analysis (LSA) of words in “Heat” and “Autodigestion (AD)” topics. Three separate word vectorizations were used; two publicly available files downloaded from MathWorks (Fast Text) and Stanford Global Word Vectorization (GloVe) (https://nlp.stanford.edu/projects/glove/), and one embedded from the TF-IDF filtered corpus of scientific articles. Cosine similarity measured the semantic relationships between all pairs of words within and between groups with values ranging from −1 to +1 (directly opposite to nearly identical, respectively). A cosine similarity of 0 indicates orthogonality. (b) Bar graph displaying the use of the “Heat” and “Autodigestion (AD)” terms in journal articles, which have become more frequently used between 2014 and the average of the last 3 years (2019–2021 with 2021 extrapolated due to the timing of this article) in all articles (top), and the number of documents that tandemly employ the two term groups have increased dramatically (normalized to the number of documents published each year). Fold change based on 2014 baseline. (c) Selected topical relationships (edges) from the network diagram in indicate that the relationships between heat stress and intestinal permeability, thermoregulation and digestive enzymes, and intestinal permeability are increasing faster than other intersecting topics examined in the current analysis
The LDA analysis efficiently underlines a prominent reference to intestinal permeability, septic shock, and inflammatory response in a large corpus of heat shock literature. In this multi-step process, we have achieved the following:
demonstration that heat illness and autodigestion topics are not often discussed in the literature to comparable lengths ();
illustration that topical relationships using network metrics to help navigate the literature (); and
interrogation of the consensus within the scientific community on the relationship between heat illness and autodigestion, as well as its trends ().
Notably, the above analysis presents an undirected network, and therefore no causal conclusions can be gleaned from it. Indeed, documents that criticize or negate the relationship between autodigestion and heat illness may also contribute to the apparent increasing discussion of this topical relationship using the given analysis presented. Perhaps a larger corpus spanning more journals and dates, as well as other topic modeling algorithms, would aid in uncovering more specific mechanistic relationships and trends. In the following section, we highlight specific evidence in selected studies that may point to a mechanistic link between these variables.
Heat stroke & autodigestion: comprehensive review of articles linking heat stress and autodigestion
The intersection of specific subject matter of interest in the literature is highly intricate. Thus, broader incorporation and analysis of literature, coupled with human semantic interpretation and insight, may be desirable in uncovering the most critical pieces of information. Hence, below we add a traditional manual keyword-based review of the literature based on 89 studies (see SM S.4) to complement the high-throughput topic modeling in Section 3.
Heat stroke and elevation of intestinal permeability
The pathogenesis of heat stress and strain has been examined within sports medicine, emergency medicine, ecological studies, and occupational health [Citation3,Citation5,Citation49–63]. Among this diversity, there is remarkable consensus on precipitating biological factors that correlate heat stress and fatal heat stroke. In general, these factors include cytokine production and leakage of endotoxins from the intestinal tract due to damaged intestinal barrier properties accompanied by a systemic inflammatory response [Citation11,Citation12,Citation14,Citation15,Citation17,Citation19,Citation21,Citation22,Citation25,Citation26,Citation29,Citation32,Citation33,Citation35–42–]. These studies underline a pathogenesis for heat stress that involves a shift in intestinal permeability for molecules of a larger size, which normally cannot permeate the intestinal barrier, to cross into the bloodstream (e.g., digestive enzymes).
A rise in intestinal permeability is caused by dysfunction of the mucosal epithelial barrier, which can occur through multiple mechanisms. A direct mechanism is structural damage of the epithelium and tight junction proteins during elevated core temperatures [Citation22,Citation25,Citation30,Citation32,Citation36–40]. Colon cell cultures exposed to heat stress (43°C) undergo lysosomal-mitochondrial pathway apoptosis with the release of Cathepsin-B. Inhibition of Cathepsin-B attenuates the breakdown of the intestinal barrier in-vivo in mice (core temperature 42°C) [Citation64]. After heating rat intestines to 42.5°C, Lambert et al. [Citation30] observed open tight junctions between intestinal epithelial cells and a breakdown of the villi in the brush border and morphologically damaged epithelium (). Further, Liu et al. [Citation22] demonstrated that as core temperatures rise above 39°C to 42°C in mice, the extent of mucosal injury in the form of epithelial necrosis and villi desquamation (or peeling) is positively correlated to core temperature. Moreover, Xiao et al. [Citation40] reported that the expression of the tight junction proteins occludin and ZO-1 in their intestinal epithelia were downregulated by 84% and 74%, respectively, after raising the core temperature of rats >41°C compared to a control group. The breakdown of the mucosal epithelial lining during elevated core temperatures can progress within an hour to complete denudation of the epithelial lining on intestinal villi (), exposing the intestinal wall to all contents in the lumen of the intestine [Citation30]. All segments of the small intestine from the duodenum to the ileum may be involved (), and heat stress has also been found to induce a significant level of apoptosis in epithelial cells, as shown in .
Figure 4. Transmission electron micrographs of small intestinal epithelial cells from one control rat and two rats heated to 42.5°C core temperature. Each image partially depicts two adjacent enterocytes. In heat-stressed rats 1 and 2, there is visible damage to the microvilli compared with the control cells. Bar represents 1 μm. Reprinted with permission from [Citation30], copyright (2002), American Physiological Society
![Figure 4. Transmission electron micrographs of small intestinal epithelial cells from one control rat and two rats heated to 42.5°C core temperature. Each image partially depicts two adjacent enterocytes. In heat-stressed rats 1 and 2, there is visible damage to the microvilli compared with the control cells. Bar represents 1 μm. Reprinted with permission from [Citation30], copyright (2002), American Physiological Society](/cms/asset/bdf5699a-e166-4da4-94d6-8347e5639ea3/ktmp_a_1922261_f0004_b.gif)
Figure 5. Representative light micrographs of H&E (Hematoxylin and Eosin) stained rat everted small intestinal sac tissue over 60 min at core temperatures of 41.5–42°C. Villi appear generally normal (barring slight subepithelial space at villous tips) at 15 min, epithelia begin sloughing from villous tips at 30 min, epithelial lining massively lift from the top and sides of villi at 45 min, and villi were completely denuded at 60 min. Bars represent 100 μm. Between 2 and 4 rats were histologically assessed at each time point. The progression of epithelial damage from the tips of villi to the base of the villi is consistent with findings from other studies. Hall et al. observed that the tips of villi retained significantly more [3H] misonidazole than the middle portion of the villi, which in turn retained significantly more than the base during heat stress Reprinted with permission from [Citation30], copyright (2002), American Physiological Society
![Figure 5. Representative light micrographs of H&E (Hematoxylin and Eosin) stained rat everted small intestinal sac tissue over 60 min at core temperatures of 41.5–42°C. Villi appear generally normal (barring slight subepithelial space at villous tips) at 15 min, epithelia begin sloughing from villous tips at 30 min, epithelial lining massively lift from the top and sides of villi at 45 min, and villi were completely denuded at 60 min. Bars represent 100 μm. Between 2 and 4 rats were histologically assessed at each time point. The progression of epithelial damage from the tips of villi to the base of the villi is consistent with findings from other studies. Hall et al. observed that the tips of villi retained significantly more [3H] misonidazole than the middle portion of the villi, which in turn retained significantly more than the base during heat stress Reprinted with permission from [Citation30], copyright (2002), American Physiological Society](/cms/asset/95590f25-2fa2-4466-a6d5-8f94615b1e22/ktmp_a_1922261_f0005_oc.jpg)
Figure 6. Representative histological images of H&E stained regions of the small intestine in exertional heat stroke (EHS) vs exercise control (EXC) mice. Images were taken 30 min after mice experienced EHS at 37.5°C air temperature or underwent equal intensity and duration of exercise at 25°C air temperature. Reprinted with permission from [Citation59], copyright (2015), American Physiological Society
![Figure 6. Representative histological images of H&E stained regions of the small intestine in exertional heat stroke (EHS) vs exercise control (EXC) mice. Images were taken 30 min after mice experienced EHS at 37.5°C air temperature or underwent equal intensity and duration of exercise at 25°C air temperature. Reprinted with permission from [Citation59], copyright (2015), American Physiological Society](/cms/asset/7114051a-cab4-426a-963c-70bd8d795998/ktmp_a_1922261_f0006_oc.jpg)
Figure 7. Effect of heat stress (HS) on the apoptosis ratio of rat intestinal epithelial cell line (IEC-6) cells. Data are reported as means ± SD, n = 6. *P < 0.01 vs control. Adapted from [Citation25]
![Figure 7. Effect of heat stress (HS) on the apoptosis ratio of rat intestinal epithelial cell line (IEC-6) cells. Data are reported as means ± SD, n = 6. *P < 0.01 vs control. Adapted from [Citation25]](/cms/asset/c80437fa-f62c-48a3-b108-3827568b8fd6/ktmp_a_1922261_f0007_b.gif)
The morphological evidence for elevated permeability is confirmed by direct estimates of intestinal permeability. Using the lactulose-to-rhamnose (L/R) ratio measurement––a method to detect the movement of a larger molecular weight and non-digestible saccharide (rhamnose with 342 Da molecular weight) across the intestinal barrier––shows a significant positive correlation with core temperature in a meta-analysis of several measurements () [Citation44]. The elevation of intestinal permeability due to rising core temperature shows individual variability between human subjects; during small elevations of core temperature, not all individuals may have elevated intestinal permeability, while at extreme core temperatures (>39°C), the majority of subjects show elevated permeability () [Citation44]). A further study found that the ileum of rats incubated at 45°C, whether in vivo or in vitro, leaks significantly more radioactively-labeled endotoxin into the surrounding medium than the ileum of non-heat stressed rats [Citation29]. Rats heated to a colonic temperature of just 41.5°C exhibit increased portal endotoxin concentrations with damage to the mucosal barrier [Citation11].
Figure 8. Correlation between core body temperature and intestinal permeability assessed with the urinary lactulose-to-rhamnose (L/R) ratio. The core body temperature and L/R ratio at baseline and at the end of exercise were obtained from the following eight studies: Pals et al. [Citation65], Lambert et al. [Citation66–68], Marchbank et al. [Citation35], Zuhl et al. [Citation69], Zuhl et al. [Citation70] and Davison et al. [Citation42]. See Pires et al. for further detail [Citation44]. Most of the plotted values are expressed as means ± standard error of the mean, while the temperature data in the study of Lambert et al. [Citation67] and the intestinal permeability data in the studies of Lambert et al. [Citation66,Citation67] are expressed as medians. Reprinted with permission from [Citation44], copyright (2016), Springer Nature
![Figure 8. Correlation between core body temperature and intestinal permeability assessed with the urinary lactulose-to-rhamnose (L/R) ratio. The core body temperature and L/R ratio at baseline and at the end of exercise were obtained from the following eight studies: Pals et al. [Citation65], Lambert et al. [Citation66–68], Marchbank et al. [Citation35], Zuhl et al. [Citation69], Zuhl et al. [Citation70] and Davison et al. [Citation42]. See Pires et al. for further detail [Citation44]. Most of the plotted values are expressed as means ± standard error of the mean, while the temperature data in the study of Lambert et al. [Citation67] and the intestinal permeability data in the studies of Lambert et al. [Citation66,Citation67] are expressed as medians. Reprinted with permission from [Citation44], copyright (2016), Springer Nature](/cms/asset/e938c3b4-83ea-48e7-9c18-75392f5c33ec/ktmp_a_1922261_f0008_oc.jpg)
Figure 9. Percentage of human participants showing increased intestinal permeability after exercise according to the core temperature levels attained, calculated by dividing the number of participants reporting augmented intestinal permeability by the total number of participants at each core temperature range as labeled in the bars. Reprinted with permission from [Citation44], copyright (2016), Springer Nature
![Figure 9. Percentage of human participants showing increased intestinal permeability after exercise according to the core temperature levels attained, calculated by dividing the number of participants reporting augmented intestinal permeability by the total number of participants at each core temperature range as labeled in the bars. Reprinted with permission from [Citation44], copyright (2016), Springer Nature](/cms/asset/242f5d83-5b0d-4136-bff7-7d591db052cb/ktmp_a_1922261_f0009_b.gif)
There is a direct association between the magnitude of hyperthermia as measured by core body temperature and the degree to which intestinal permeability increases. Pires et al. [Citation44] show that intestinal hyperpermeability in humans begins to occur at core temperatures above 38.5°C and is almost certain to occur at core temperatures above 39°C. Furthermore, it has been found that increasing core temperature accounts for 63% of the variance in intestinal permeability [Citation44]. Although intestinal permeability is often observed when the core temperature exceeds 39°C during exercise, it should be noted that well-conditioned individuals, such as athletes, commonly reach temperatures higher than 39°C while training and/or competing [Citation71,Citation72] yet the incidence of heat stroke is relatively low. Moreover, enhanced intestinal permeability does not always lead to heat illness, especially in light of the liver’s capacity to filter out many constituents from portal venous blood.
Exertional heat stroke (versus classic) can also lead to different physiological responses between individuals. The sensitivity of intestinal permeability to an elevation in core temperature is reported to depend on co-factors related to health, such as the level of training in healthy individuals and athletes, and conversely, risk factors of age, diabetes, hypertension, etc., in individuals with co-morbidities. This sensitivity is illustrated by the significantly elevated presence of plasma endotoxin in the circulation of trained versus untrained healthy adults () during exertional heat stress [Citation19]. Circulating plasma endotoxin is a further measure of elevated intestinal permeability––as paracellular pathways between epithelial cells open, contents of the small intestine, including bacteria and endotoxin such as lipopolysaccharides, appear in the portal circulation [Citation11,Citation12,Citation29,Citation73]. In humans, exercise-induced gastrointestinal syndrome (EIGS) can display with impaired nutrient absorption, immune surveillance, and endotoxemia. The impact of strenuous exercise on intestinal injury can be assessed by measuring the release of intestinal fatty acid binding protein (I-FABP) from the intestinal mucosa into circulation. Exercise in higher ambient temperatures significantly increases levels of I-FABP in humans [Citation74]. Similarly, rats that exercise in warmer environments (31°C) exhibit increased intestinal permeability to radiolabeled 99mTc-diethylenetriaminepentaacetic acid (99mTc-DTPA) compared to cooler environments (13°C–24°C) and display heightened mRNA levels of tight junction related genes in what is hypothesized to be an attenuated attempt to palliate the increased intestinal permeability, but the proteins’ expressions were not directly measured in this study [Citation75]. Such findings highlight the individual physiological status determining heat stress response. Furthermore, no significant sex disparity in the susceptibility to increased intestinal permeability to exertional and ambient heat stresses has yet been noted [Citation34]. However, enhanced intestinal permeability does not equate to heat stress or the potentially deadly morbidities discussed here. Given the general health benefits of exercise, the potential impacts of enhanced permeability are unlikely to outweigh the benefits of exercise and its positive health adaptations [Citation34], yet the enhanced intestinal permeability does not equate to heat stress. Further, given the general health benefits of exercise, the potential impacts of enhanced permeability are unlikely to outweigh the benefits of exercise and its positive health adaptations [Citation76], yet those exercising in the heat should remain cognizant, be prepared, allow acclimatization, and respect the dangers of extreme heat. Further documentation of these dangers is available through several resources [Citation77–79].
Figure 10. Circulating plasma endotoxin concentrations in trained (TR) and untrained (UT) groups of healthy adult men during exertional heat stress (EHS). Values are means ± SE. From baseline to 38.5°C core temperature (Tc) and exhaustion (Exh), n = 12 TR and 11 UT; at 39.0°C Tc, n = 12 TR and 9 = UT; and at 39.5°C Tc, n = 11 TR. *P < 0.05, UT significantly different from baseline. †P < 0.05, TR significantly different from baseline. ‡P < 0.05, UT significantly different from baseline to 38.5°C Tc. §P < 0.05, between-group significance. Reprinted with permission from [Citation19], copyright (2008), American Physiolgical Society
![Figure 10. Circulating plasma endotoxin concentrations in trained (TR) and untrained (UT) groups of healthy adult men during exertional heat stress (EHS). Values are means ± SE. From baseline to 38.5°C core temperature (Tc) and exhaustion (Exh), n = 12 TR and 11 UT; at 39.0°C Tc, n = 12 TR and 9 = UT; and at 39.5°C Tc, n = 11 TR. *P < 0.05, UT significantly different from baseline. †P < 0.05, TR significantly different from baseline. ‡P < 0.05, UT significantly different from baseline to 38.5°C Tc. §P < 0.05, between-group significance. Reprinted with permission from [Citation19], copyright (2008), American Physiolgical Society](/cms/asset/53d41c95-1c1c-44ab-b84a-33a09bbd0550/ktmp_a_1922261_f0010_b.gif)
Blood flow reduction and intestinal permeability
The reduction of blood flow to the intestine has a direct impact on intestinal permeability. This effect is shown in hemorrhagic shock with a global reduction of blood flow due to central arterial blood pressure reduction or local reduction of blood flow after splanchnic/mesenteric artery occlusions. The reduced perfusion leads to hypoxia in the epithelial cells, opening of intercellular junctions, apoptosis, and consequently elevated permeability of the epithelium [Citation11,Citation12,Citation14,Citation25,Citation34,Citation43,Citation73]. Elevated core temperatures, through exertional heat generation and/or environmental heat exposure, also result in blood flow reduction in the visceral organs [Citation34,Citation43,Citation44]. For example, rats that were heated such that their colonic temperature rose from 37°C to 41.5°C exhibit ~40% decrease in splanchnic blood flow [Citation12].
In human studies, males subjected to whole-body heating for over 40 minutes had on average a 40% reduction of splanchnic blood flow, as detected by infusion and hepatic extraction of indocyanine green dye, while blood flow to the skin increased by 1 L min−1 [Citation80]. Similarly, volunteers subjected to a moderate-to-severe exercise condition with no ambient heating experienced a splanchnic blood flow reduction of 50–75% from a resting flow rate of 1.61 liters/min. The heat-induced reduction of blood flow occurs under both classic and exertional heat stress [Citation80].
The neuronal pathways that control intestinal perfusion and permeability may also play a role in heat stress. Damages to the central nervous system (CNS), such as by disruption of the hypothalamic-pituitary-adrenal axis [Citation24] or the parasympathetic innervation of the intestine [Citation81], affect thermoregulation and heat stress. For example, as a consequence of an elevated core temperature and altered blood distribution, cerebral blood flow is reduced, leading to cerebral hypoxia and even intracranial hemorrhage with permanent brain damage [Citation82]. The parasympathetic innervation of the intestine plays a central role in intestinal peristalsis and food transport in the small intestine [Citation83]. Its interruption causes stagnation (of partially digested boluses and their digestive enzymes) during peristaltic propulsion along the small intestine, a situation that can lead to disruption of the epithelial barrier and locally elevated mucosal barrier permeability [Citation84].
Heat stress and cytokines
Hyperthermia-induced elevated intestinal permeability is accompanied by an extensive body of evidence that documents not only the appearance of inflammatory mediators, such as endotoxin, but also the presence of inflammatory signaling molecules, such as oxygen radicals, cytokines, and chemokines. The rise of endotoxin concentration in the portal circulation after exposure of rats to 40°C ambient temperature is accompanied by increased levels of the radicals ceruloplasmin, semiquinone, and heme-NO, resulting in an approximate doubling of the endotoxin concentration in the portal circulation [Citation12]. Mice whose core temperatures are raised to 39, 40, 41, and 42°C showed significant correlations between intestinal levels of the cytokines IL-1β, IL-10, and IL-12p40, as well as intestinal injury scores [Citation22]. Exertional heat stress has shown similar permeability and IL-6 signaling in humans [Citation85]. Virtually all human diseases are accompanied by inflammatory signaling molecules, irrespective of the nature of the original injury. During heat strain, no specific time course of a combination of cytokines or chemokines has been identified. Since the inflammatory cascade is fundamentally a tissue repair mechanism [Citation86], the evidence may indicate that heat directly injures tissue and triggers the inflammatory repair cascade, much like other injuries will do so. The details of the inflammatory repair cascade in heat stress remain to be clarified.
Human studies in heat stroke patients or healthy volunteers exposed to exertional heat stress are largely observational with relatively low sample sizes. Despite such limitations, findings similar to the animal studies are reported: humans experiencing elevated core temperatures exhibit elevated blood levels of serum endotoxins as well as cytokines [Citation8–10,Citation15,Citation18–20,Citation35,Citation42], indicating increased intestinal permeability with inflammation (). Systematic studies of the intestinal mucosal barriers, whether in heat stroke patients or healthy volunteers exposed to heat stress, are required.
Figure 11. Correlation of lipopolysaccharide (LPS), tumor necrosis factor (TNF), and interleukin 1 (IL-1) with rectal temperature during heat stroke. Data points represent measurements taken from 17 adult patients with mean rectal temperatures between 40.6 and 43.3°C. Reprinted with permission from [Citation9], copyright (1991), American Physiological Society
![Figure 11. Correlation of lipopolysaccharide (LPS), tumor necrosis factor (TNF), and interleukin 1 (IL-1) with rectal temperature during heat stroke. Data points represent measurements taken from 17 adult patients with mean rectal temperatures between 40.6 and 43.3°C. Reprinted with permission from [Citation9], copyright (1991), American Physiological Society](/cms/asset/14eba0de-66a0-4949-9a97-c4c403df4be4/ktmp_a_1922261_f0011_b.gif)
Heat stress and multi-organ failure
The sequence of events that lead to multiple-organ dysfunction and fatal heat stroke is uncertain. Elevated core temperatures can be directly injurious to cells and organs. However, besides such a direct thermal mechanism, there is a need to analyze the progression of delayed cell and organ injuries after a heat exposure that may occur even after the restoration of normal body temperatures. Such research may open new opportunities for individual-level interventions.
Whereas elevated levels of cytokines in the circulation correlate with the severity of heat stress [Citation9,Citation10,Citation16], these studies provide limited mechanistic insight into the actual cell or organ dysfunctions. There is no conclusive evidence that cytokines per se cause multiorgan failure or increased intestinal permeability. Instead, as mentioned above, they may be part of the inflammatory cascade that serves as the tissue repair mechanism following cell and organ injury in heat stroke [Citation22,Citation23,Citation27]. In a similar situation, endotoxemia that stimulates the release of cytokines [Citation13] can lead to multiorgan failure and death [Citation8,Citation21,Citation40]. However, there is no conclusive evidence that endotoxin alone, without additional steps, is responsible for multiorgan failure. Endotoxin may be responsible for the elevated intestinal permeability (e.g., via the toll-like receptors (TLR-4) pathway [Citation87]), but its signaling pathway in shock is uncertain as no interventions against either endotoxin or its receptors have led to a clinically-approved intervention in patients.
Autodigestion and heat stroke morbidities
A potential missing link between elevated intestinal permeability and fatal heat stroke is the leakage of digestive enzymes across the injured mucosal barrier, which has not yet been elucidated in the current literature on heat stress and strain in humans. Digestive enzymes are generated in the pancreas and released into the lumen of the upper (duodenal) segment of the small intestine. The enzymes are retained inside the lumen of the small intestine so that the mixture of food and digestive enzymes can continue to react while being carried via peristaltic motion along the intestine. The pancreatic proteases, amylases, lipases, and nucleases are powerful since they are concentrated, fully activated, and have relatively nonspecific activity. They can degrade most biomolecules, including one’s own intestine, and they generate inflammatory mediators during food degradation [Citation88].
In light of the evidence that endotoxin frequently forms aggregates >1,000 kDa in size, which is larger than digestive enzymes (with molecular weight ~ 20 to 30 kDa), one is justified to hypothesize that digestive enzymes cross the mucosal barrier during exposure to elevated core temperatures. This connection may be a potential cause of the multiple-organ failure in fatal heat stroke, as outlined below.
The intestinal tract is well-recognized to play a central role in disease, yet the molecular/cellular mechanisms that give it this particular distinction are only recently surfacing. Among several constituents in the intestine, such as bacteria, viruses, fungi, and inorganic nanoparticles, we focus here on the pancreatic digestive enzymes––the key players in digestion.
Since the intestine continually degrades large volumes of biomolecules from food, it needs special protective mechanisms to guard against digesting autologous tissue, i.e., autodigestion. Autodigestion is prevented by restricting digestive enzymes to the lumen of the small intestine [Citation89]. The compartmentalization of digestive enzymes is facilitated by the mucosal barrier consisting of specialized columnar cells covered by a lamina of highly glycosylated gel-forming mucus. This protective layer is comprised of several members of the mucin family, some attached to the luminal side of the epithelial cells’ microvilli, forming the glycocalyx that parries digestive enzymes, and some secreted from goblet cells between the epithelium [Citation90]. With this sheath of glycoproteins, the pancreatic proteases are unable to reach any peptide bonds, as the required glycosidases are not endogenous to the intestine. Thus, the mucin layer is an important diffusion barrier that prevents molecules the size of digestive enzymes from passing into the mucosal layer and various wall layers of the intestine [Citation91]. The mucin does, however, allow nutrients (ions, peptides, etc.) to pass for absorption by the epithelium as an essential part of nutrient delivery.
Recent evidence shows that the breakdown of this mucosal barrier, which can be exacerbated by elevated core temperatures, can result in the escape of digestive enzymes, a process with potentially severe consequences. For example, in intestinal ischemia, the mucosal barrier is breached and digestive enzymes escape into the submucosal tissue. During this passage, the enzymes digest the intestinal villi exposing intestinal tissue unprotected to its own digestive enzymes [Citation92]. The digestive enzymes are further carried into the systemic circulation through the portal venous circulation, the intestinal lymphatics, and even via the peritoneum. These enzymes activate secondary degrading enzymes, e.g., matrix metalloproteinases [Citation92,Citation93], and generate cytotoxic mediators, such as lipid degradation products (e.g., unbound free fatty acids) [Citation94,Citation95].
One of the consequences of such unchecked protease activity is the cleavage of membrane receptors, a process that compromises the associated cell functions they control. For example, cleavage of the adrenergic receptors may cause a central blood pressure reduction [Citation96], an elevated permeability to molecules the size of digestive enzymes as interepithelial membrane adhesion molecules (e.g., E-cadherin) are cleaved [Citation91,Citation97], or insulin resistance may arise as the ectodomain of the insulin receptor is cleaved [Citation98]. While to date, receptor cleavage has not been investigated in heat shock, it is notable that post-exertional heat stroke and even post-exercise controls exhibit hyperglycemia over several hours and days (). One exception is the cleavage and activation of the protease-activated receptor (PAR1). Pharmacological inhibition of this receptor attenuates elevation of the intestinal permeability after heat stress ().
Figure 12. Blood glucose levels in mice taken at various time points into recovery post-exertional heat stroke (EHS) or -exercise control (EXC) compared with a naive control group (NC). A: 0.5 h post-EHS or -EXC. B: 3 h post-EHS or -EXC. C: 24 h post-EHS or -EXC. D: 4 days post-EHS; glucose tests were only available for EHS groups receiving 30% and 90% relative humidity at 37.5°C ambient temperature. Data are medians ± 25–75% ranges. *P < 0.05, **P < 0.01. Reprinted with permission from [Citation59], copyright (2015), American Physiological Society
![Figure 12. Blood glucose levels in mice taken at various time points into recovery post-exertional heat stroke (EHS) or -exercise control (EXC) compared with a naive control group (NC). A: 0.5 h post-EHS or -EXC. B: 3 h post-EHS or -EXC. C: 24 h post-EHS or -EXC. D: 4 days post-EHS; glucose tests were only available for EHS groups receiving 30% and 90% relative humidity at 37.5°C ambient temperature. Data are medians ± 25–75% ranges. *P < 0.05, **P < 0.01. Reprinted with permission from [Citation59], copyright (2015), American Physiological Society](/cms/asset/d6c6ac01-a079-4c57-8707-5d2cd167d181/ktmp_a_1922261_f0012_b.gif)
Figure 13. Effects of heat stress and protease-activated receptor 1 (PAR1) inhibitor RWJ58,259 on mouse intestinal permeability as assessed with the urinary lactulose-to-rhamnose (L/R) ratio. The heat stressed (HS) and HS + RWJ58,259 mice had core temperatures of 41°C and recovered at room temperature for 24 h. In the HS + RWJ-58259 group, mice were injected with RWJ-58259 (10 mg/kg) via a tail vein 30 min before heat stress. In the Control group, the mice were injected with a vehicle solution and left at room temperature for the same amount of time. Data are mean ± SD. *P < 0.05 vs Control, #P < 0.05 vs HS, n = 6–8. Reprinted with permission from [Citation41]
![Figure 13. Effects of heat stress and protease-activated receptor 1 (PAR1) inhibitor RWJ58,259 on mouse intestinal permeability as assessed with the urinary lactulose-to-rhamnose (L/R) ratio. The heat stressed (HS) and HS + RWJ58,259 mice had core temperatures of 41°C and recovered at room temperature for 24 h. In the HS + RWJ-58259 group, mice were injected with RWJ-58259 (10 mg/kg) via a tail vein 30 min before heat stress. In the Control group, the mice were injected with a vehicle solution and left at room temperature for the same amount of time. Data are mean ± SD. *P < 0.05 vs Control, #P < 0.05 vs HS, n = 6–8. Reprinted with permission from [Citation41]](/cms/asset/ec057407-66ae-404d-9eba-76f8145c6206/ktmp_a_1922261_f0013_b.gif)
Enzymatic degradation of plasma proteins leading to accumulation of peptidic fragments by degradation results from unchecked protease activity in the circulation [Citation99]. Whereas the combination of these cytotoxic products and reactions can lead to death within hours, an enteral blockade of digestive enzymes can attenuate the autodigestion and reduce the breakdown of the mucosal barrier, prevent multiple forms of organ dysfunction, as well as prevent mortality [Citation84].
Maintenance of the mucosal barrier is an ATP-consuming process that can be fulminant disrupted. Examples include reducing oxygen or glucose supply or the presence of inflammatory mediators, such as endotoxin [Citation84,Citation97]. A key question arises in the current analysis––can a core temperature rise (or drop) in the intestine from its homeostatic control values cause a breakdown of the mucosal barrier and thereby precipitate autodigestion and co-morbidities? Epithelial permeability is temperature-sensitive [Citation32,Citation100], just like endothelial permeability [Citation41], yet permeability measurements concerning digestive enzymes in intestinal epithelium remain to be documented.
While inflammatory mediators (e.g., endotoxin) have been recorded repeatedly, their role in multiorgan failure may be indirect; they elevate intestinal permeability, causing digestive enzyme leakage. Enteral pancreatic enzyme blockade in the small intestine during endotoxemia attenuates multiorgan failure and cytokine markers for inflammation [Citation84,Citation101], yet significant gaps remain in understanding the mechanisms that mediate multiorgan failure and heat stress [Citation16].
Public health & epidemiology
Potential public health implications of this research include advancing the fundamental understanding around specific intrinsic risk factors that limit one’s ability to rapidly resolve SIRS following heat stroke, such as poor health, preexisting illness, drug and alcohol use, compromised digestion, age-related impacts to the intestinal tract, and concurrent exposures, among other related concerns highlighted in this discussion. The given literature reviews saw minimal overlap between public health or epidemiological studies connected to intestinal permeability, SIRS, and/or multiorgan failure caused by elevated core temperatures. Increasing concerns surrounding extreme heat exposure, climate change, urban growth, and increasingly vulnerable populations (whether due to low adaptive capacity and/or intrinsic risk factors) [Citation2,Citation102] supports advanced systematic assessments of the direct relationships between core temperature, intestinal integrity, digestive enzymes, SIRS, and ensuing multiorgan failure among individuals affected by heat stroke. With an aging population and increasing co-morbidities within the general population globally [Citation103], advanced testing of this hypothesis may save lives and support improved methods for the clinical identification or treatment of heat stroke victims.
Conclusions
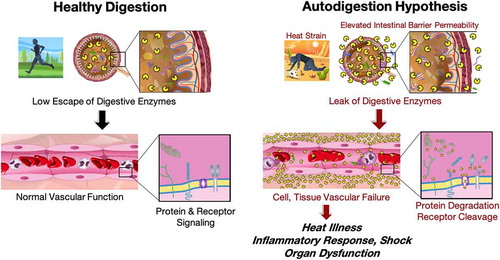
Heat stroke is multi-step, life-threatening, and can have long-term consequences to survivors due to SIRS. Significant gaps remain in understanding the pathophysiology of heat stroke. This literature summary examines a possible relationship between heat stress and autodigestion co-morbidities and interrogates its trends in a large body of scientific literature. An unbiased machine-based search of the literature indicates that heat illness and autodigestion have not been discussed in the literature to any extend. The presence of a concomitant intestinal permeability related topical class indicates that heat stress-mediated organ injury may depend on a pathway involving the small intestine barrier and an elevation of its mucosal epithelial permeability to molecules the size of digestive enzymes. The leakage of these enzymes from the intestinal tract into the systemic circulation may be related to SIRS and sepsis (). The current evidence outlined in the two literature summaries presented here is compatible with this possibility, yet critical elements of the hypothesis remain to be tested.
Figure 14. Summary of a hypothesis that links heat strain, elevated intestinal permeability to leak of digestive enzymes from the lumen of the intestine into the systemic circulation. During healthy digestion (left) digestive enzymes are compartmentalized within the small intestine. After heat stress (left) they leak into the systemic circulation, cause cell and tissue failure by cleavage of plasma proteins and membrane receptors, heat illness, an inflammatory response, shock and organ dysfunctions. Drawings courtesy of Peter J. Schmid-Schoenbein.
Direct measurements of digestive enzymes and their activity in plasma of patients during heat shock are required [Citation104], and it remains to be examined whether interventions to block these enzymes serve to attenuate the co-morbidities following heat exposure. Digestive enzymes are always present in specific areas of the body and relatively nonspecific. Their accumulation outside the compartmentalization of the pancreas and the intestine epithelial barrier leads to relatively nonspecific degradation, as is the case in normal food digestion. Multiple cell functions can be impaired by proteases that degrade extracellular domains of membrane receptors required for these cell functions. A reassessment and new advances are needed to elucidate the linkages of endotoxins and cytokines with heat stroke and identify the role of these processes in SIRS as related to growing public health concerns around heat exposure, aging, co-morbidities, and other vulnerabilities. Enhanced collaboration and translation between disciplines (medicine, public health, occupational health, physiology, bioengineering) may also advance how information is used and how real-world data are collected to improve understanding of the pathophysiology involved in heat stroke.
Abbreviations
| AD – | = | Autodigestion |
| BMI – | = | Body Mass Index |
| CNS – | = | Central Nervous System |
| ECOC – | = | Error Correcting Output Codes |
| EHS – | = | Exertional Heat Stroke |
| EIGS – | = | Exercise Induced Gastrointestinal Syndrome |
| EXC – | = | Exercise Control |
| Exh – | = | Exhaustion |
| GloVe – | = | Global Word Vectorization |
| HS – | = | Heat Stress(ed) |
| IEC-6 – | = | Intestinal Epithelial Cell |
| I-FABP – | = | Intestinal Fatty Acid Binding Protein |
| IL-1 – | = | Interleukin 1 |
| IL-1β – | = | Interleukin 1 Beta |
| IL-6 – | = | Interleukin 6 |
| IL-10 – | = | Interleukin 10 |
| IL-12p40 – | = | Interleukin 12 subunit p40 |
| LDA – | = | Latent Dirichlet Allocation |
| LPS – | = | Lipopolysaccharide |
| L/R – | = | Lactulose-to-Rhamnose |
| LSA – | = | Latent Semantic Analysis |
| PAR1 – | = | Protease-Activated Receptor |
| SIRS – | = | Systemic Inflammatory Syndrome |
| SM – | = | Supplemental Material |
| SVM – | = | Support Vector Machines |
| Tc – | = | Core Temperature |
| TF-IDF – | = | Term-Frequency Inverse Document-Frequency |
| TLR-4 – | = | Toll-Like Receptors 4 |
| TNF – | = | Tumor Necrosis Factor |
| TR – | = | Trained |
| UT – | = | Untrained |
| 99mTc-DTPA – | = | 99mTc-diethylenetriaminepentaacetic |
Disclosure
AAF, AZ, and JKV declare no conflict of interest. GWSS owns stock in Leading Biosciences, Inc., a company to develop shock treatments.
Supplemental Material
Download PDF (398 KB)Supplementary material
Supplemental data for this article can be accessed here
Data availability statement
Please contact Anthony A. Fung ([email protected]) for the corpus code, which is comprised of commercial MATLAB toolboxes, as well as network diagrams made in Gephi.
Additional information
Notes on contributors
Anthony A. Fung
Anthony A. Fung is PhD student in the department of Bioengineering at the University of California, San Diego. His research is focused on biomedical applications of Raman Scattering Microscopy in ageing and metabolism. Anthony is also developing novel and user-friendly tools for scientists and clinicians to adopt Raman technology at all stages of research and development. He is a founding member of the Design For America studio at UC San Diego, and former team lead of award-winning Global TIES projects.
Andy Zhou
Andy Zhou is a Master’s student in the Department of Electrical and Computer Engineering at Carnegie Mellon University. His technical interests include machine learning, distributed systems, and cloud computing.
Jennifer K. Vanos
Dr. Jennifer Vanos holds an interdisciplinary appointment studying climate and human health in the School of Sustainability at Arizona State University. As a human biometeorologist, she works to strengthen the understanding and practice surrounding how we protect people from extreme heat in a world impacted by climate change. Her work on extreme heat addresses risks and challenges within varying vulnerable populations, including children, athletes, and outdoor workers. She approaches her work using various measurement and modeling tools and frameworks across spatiotemporal scales. She is a member of ASU’s Urban Climate Research Center and currently Chair of the American Meteorological Society’s Board on Environment & Health. She previously worked at UC San Diego, Texas Tech University, Health Canada, and the University of Guelph.
Geert W. Schmid-Schönbein
Geert W. Schmid-Schönbein is Distinguished Professor and former Chair of the Department of Bioengineering at the University of California in San Diego which is where he received his graduate degree. He conducted postdoctoral research at Columbia University after which he returned to the University of California in San Diego. His research is focused on blood flow in the microcirculation, cell mechanics and mechanotransduction with application to human disease. His team uncovered a previously unknown mechanism for inflammation due to “Auto-digestion”. He is Founding Member of American Institute of Medical and Biological Engineering, former President of the Biomedical Engineering Society, the Microcirculatory Society and the North American Society of Biorheology. He was Chair of the World Council for Biomechanics and the US National Committee for Biomechanics and is a member of the National Academy of Engineering.
References
- Krayenhoff ES, Moustaoui M, Broadbent AM, et al. Diurnal interaction between urban expansion, climate change and adaptation in US cities. Nat Clim Change. 2018;8(12):1097–1103.
- Leon LR, Bouchama A. Heat stroke. Compr Physiol. 2015;5(2):611–647.
- Eichner E. Treatment of suspected heat illness. Int J Sports Med. 1998;19:S150–S153.
- Coris EE, Ramirez AM, Van Durme DJ. Heat illness in athletes: the dangerous combination of heat, humidity and exercise. Sports Med. 2004;34(1):9–16.
- Howe AS, Boden BP. Heat-related illness in athletes. Am J Sports Med. 2007;35(8):1384–1395.
- Gonzalez RR, Halford C, Keach EM. Environmental and physiological simulation of heat stroke: a case study analysis andvalidation. J Therm Biol. 2010;35(8):441–449.
- Jay O, Brotherhood JR. Occupational heat stress in Australian workplaces. Temperature. 2016;3(3):394–411. doi: https://doi.org/10.1080/23328940.2016.1216256.
- Graber CD, Reinhold RB, Breman JG. Fatal heat stroke: circulating endotoxin and gram-negative sepsis as complications. J Am Med Assoc. 1971;216(7):1195–1196.
- Bouchama A, Parhar RS, El-yazigi A, et al. Endotoxemia and release of tumor necrosis factor and interleukin 1a in acute heatstroke. J Appl Physiol. 1991;70(6):2640–2644. (1985).
- Bouchama A, Al-Sedairy S, Siddiqui S, et al. Elevated pyrogenic cytokines in heatstroke. Chest. 1993;104(5):1498–1502.
- Hall DM, Baumgardner KR, Oberley TD, et al. Splanchnic tissues undergo hypoxic stress during whole body hyperthermia. Am J Physiol. 1999;276(5):G1195–G1203.
- Hall DM, Buettner GR, Oberley LW, et al. Mechanisms of circulatory and intestinal barrier dysfunction during whole body hyperthermia. Am J Physiol. 2001;280(2):H509–H521.
- DuBose D, Balcius J, Morehouse D. Heat stress and/or endotoxin effects on cytokine expression by human whole blood. Shock. 2002;17(3):217–221.
- Han X, Fink MP, Uchiyama T, et al. Increased iNOS activity is essential for hepatic epithelial tight junction dysfunction in endotoxemic mice. Am J Physiol Gastrointest Liver Physiol. 2003;286(1):G126–136.
- Aibiki M, Ohtsubo S, Nishiyama T, et al. Elevated serum beta-D-glucan level and depressed neutrophil phagocytosis in a heatstroke patient. Resuscitation. 2005;65(1):115–117.
- Leon LR. The thermoregulatory consequences of heat stroke: are cytokines involved? J Therm Biol. 2006;31(1–2):67–81.
- Lim CL, Wilson G, Brown L, et al. Pre-existing inflammatory state compromises heat tolerance in rats exposed to heat stress. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R186–94.
- Huisse M-G, Pease S, Hurtado-Nedelec M, et al. Leukocyte activation: the link between inflammation and coagulation during heatstroke. A study of patients during the 2003 heat wave in Paris. Crit Care Med. 2008;36(8):2288–2295.
- Selkirk GA, McLellan TM, Wright HE, et al. Mild endotoxemia, NF-kappaB translocation, and cytokine increase during exertional heat stress in trained and untrained individuals. Am J Physiol Regul Integr Comp Physiol. 2008;295(2):R611–23.
- Chang DM. The Role of Cytokines in Heat Stroke. Immunol Invest. 2009;22(8):553–561.
- Lin X, Li Y-J, Li Z-L, et al. Pre-existing Lipopolysaccharide may increase the risk of heatstroke in rats. Am J Med Sci. 2009;337(4):265–270.
- Liu Z, Sun X, Tang J, et al. Intestinal inflammation and tissue injury in response to heat stress and cooling treatment in mice. Mol Med Rep. 2011;4(3):437–443.
- Welc SS, Phillips NA, Oca-Cossio J, et al. Hyperthermia increases interleukin-6 in mouse skeletal muscle. Am J Physiol Cell Physiol. 2012;303(4):C455–66.
- Chen S, Lin M, Chang C. Ischemic and Oxidative damage to the hypothalamus may be responsible for heat stroke. Curr Neuropharmacol. 2013;11(2):129–140.
- Tang J, Jiang Y, Tang Y, et al. Effects of propofol on damage of rat intestinal epithelial cells induced by heat stress and lipopolysaccharides. Braz J Med Biol Res. 2013;46(6):507–512.
- Chen XJ, Tang -Z-Z, Zhu -G-G, et al. JNK signaling is required for the MIP1alphaassociated regulation of Kupffer cells in the heat stroke response. Mol Med Rep. 2017;16(3):2389–2396.
- King MA, Leon LR, Morse DA, et al. Unique cytokine and chemokine responses to exertional heat stroke in mice. J Appl Physiol. 2017;122(2):296–306. (1985).
- Schmid-Schönbein GW. 2008 Landis Award lecture. Inflammation and the autodigestion hypothesis. Microcirculation. 2009;16(4):289–306.
- Shapiro Y, Alkan M, Epstein Y, et al. Increase in rat intestinal permeability to endotoxin during hyperthermia. Eur J Appl Physiol Occup Physiol. 1986;55(4):410–412.
- Lambert GP, Gisolfi CV, Berg DJ, et al. Selected Contribution: hyperthermia-induced intestinal permeability and the role of oxidative and nitrosative stress. J Appl Physiol. 2002;92(4):1750–1761. (1985).
- Lambert GP. Role of gastrointestinal permeability in exertional heatstroke. Exerc Sport Sci Rev. 2004;32(4):185–190.
- Dokladny K, Moseley PL, Ma TY. Physiologically relevant increase in temperature causes an increase in intestinal epithelial tight junction permeability. Am J Physiol Gastrointest Liver Physiol. 2006;290(2):G204–12.
- Singleton KD, Wischmeyer PE. Oral glutamine enhances heat shock protein expression and improves survival following hyperthermia. Shock. 2006;25(3):295–299.
- Lambert GP. Intestinal barrier dysfunction, endotoxemia, and gastrointestinal symptoms: the ‘canary in the coal mine’ during exercise-heat stress? Thermoregulation Hum Perform. 2008;53:61–73.
- Marchbank T, Davison G, Oakes JR, et al. The nutriceutical bovine colostrum truncates the increase in gut permeability caused by heavy exercise in athletes. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G477–84.
- Smith RM, Erlwanger KH, Rogers GG. The effect of a single dose of tumor necrosis factor alpha inhibitor on gut permeability in rats during exposure to a heat stress. J Therm Biol. 2012;37(2):151–158.
- Pearce SC, Mani V, Boddicker RL, et al. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS One. 2013;8(8):e70215.
- Xiao G, Tang L, Yuan F, et al. Eicosapentaenoic acid enhances heat stress-impaired intestinal epithelial barrier function in Caco-2 cells. PLoS One. 2013;8(9):e73571.
- Soares ADN, Costa KA, Wanner SP, et al. Dietary glutamine prevents the loss of intestinal barrier function and attenuates the increase in core body temperature induced by acute heat exposure. Br J Nutr. 2014;112(10):1601–1610.
- Xiao G, Yuan F, Geng Y, et al. Eicosapentaenoic acid enhances heatstroke-impaired intestinal epithelial barrier function in rats. Shock. 2015;44(4):348–356.
- Xu Q-L, Guo X-H, Liu J-X, et al. Blockage of protease-activated receptor 1 ameliorates heat-stress induced intestinal high permeability and bacterial translocation. Cell Biol Int. 2015;39(4):411–417.
- Davison G, Marchbank T, March DS, et al. Zinc carnosine works with bovine colostrum in truncating heavy exercise-induced increase in gut permeability in healthy volunteers. Am J Clin Nutr. 2016;104(2):526–536.
- Gupta A, Chauhan NR, Chowdhury D, et al. Heat stress modulated gastrointestinal barrier dysfunction: role of tight junctions and heat shock proteins. Scand J Gastroenterol. 2017;52(12):1315–1319.
- Pires W, Veneroso CE, Wanner SP, et al. Association between exercise-induced hyperthermia and intestinal permeability: a systematic review. Sports Med. 2017;47(7):1389–1403.
- Blei DM, Ng AY, Jordan MI. Latent dirichlet allocation. J Mach Learn Res. 2003;3:993–1022.
- Srivastava A, Sahami M. Text mining: classification, clustering, and applications. Vol. 328. Chapman and Hall; 2009. Boca Raton: Taylor and Francis CRC Group.
- Liu E TF-IDF, term frequency-inverse document frequency. 2015; [cited 2020 Sept 30]. Available from: https://ethen8181.github.io/machine-learning/clustering_old/tf_idf/tf_idf.html
- Dietterich TG, Bakiri G. Solving multiclass learning problems via error-correcting output codes. Journal of Artificial Intelligence Research. 1995;2:263–286.
- Glazer JL. Management of heatstroke and heat exhaustion. Am Fam Physician. 2005;71(11):2133–2140.
- Aggarwal Y, Karan BM, Das BN, et al. Backpropagation ANN-based prediction of exertional heat illness. J Med Syst. 2007;31(6):547–550.
- Hollowell DR. Perceptions of, and reactions to, environmental heat: a brief note on issues of concern in relation to occupational health. Glob Health Action. 2010;3.
- Leon LR, Helwig BG. Heat stroke: role of the systemic inflammatory response. J Appl Physiol. 2010;109(6):1980–1988. (1985).
- Tsai M-K, Chen I-H, Wang -C-C, et al. Colon perforation as a critical complication of exertional heat stroke. Intern Med. 2010;49(22):2473–2476.
- Zeller L, Novack V, Barski L, et al. Exertional heatstroke: clinical characteristics, diagnostic and therapeutic considerations. Eur J Intern Med. 2011;22(3):296–299.
- Horseman MA, Rather-Conally J, Saavedra C, et al. A case of severe heatstroke and review of pathophysiology, clinical presentation, and treatment. J Intensive Care Med. 2013;28(6):334–340.
- Jordan K. A 34-year-old triathlete with hyperthermia. J Emerg Nurs. 2013;39(6):623–624.
- Epstein Y, Roberts WO, Golan R, et al. Sepsis, septic shock, and fatal exertional heat stroke. Curr Sports Med Rep. 2015;14(1):64–69.
- Garber JB, Saile K, Rademacher N, et al. Pneumothorax in a dog caused by necrotizing pneumonia secondary to heatstroke. J Vet Emerg Crit Care (San Antonio). 2015;25(6):759–764.
- King MA, Leon LR, Mustico DL, et al. Biomarkers of multiorgan injury in a preclinical model of exertional heat stroke. J Appl Physiol. 2015;118(10):1207–1220. (1985).
- Cheshire WP Jr. Thermoregulatory disorders and illness related to heat and cold stress. Auton Neurosci. 2016;196:91–104.
- Deshwal R, Tiwari D, Singh R. Clinical and biochemical characteristics of exertional heat stroke among paratroopers in Agra, India. J Assoc Physicians India. 2017;65(2):57–61.
- Armstrong LE, Lee EC, Armstrong EM. Interactions of gut microbiota, endotoxemia, immune function, and diet in exertional heatstroke. J Sports Med (Hindawi Publ Corp). 2018;2018:5724575.
- Oh RC, Malave B, Chaltry JD. Collapse in the heat - from overhydration to the emergency room - three cases of exercise-associated hyponatremia associated with exertional heat illness. Mil Med. 2018;183(3–4):e225–e228.
- Yi G, Li L, Luo M, et al. Heat stress induces intestinal injury through lysosome- and mitochondria-dependent pathway in vivo and in vitro. Oncotarget. 2017;8(25):40741–40755.
- Pals KL, Chang R-T, Ryan AJ, et al. Effect of running intensity on intestinal permeability. J Appl Physiol. 1997;82(2):571–576.
- Lambert GP, Lang J, Bull A, et al. Fluid restriction during running increases GI permeability. Int J Sports Med. 2008;29(3):194–198.
- Lambert GP, Boylan M, Laventure J-P, et al. Effect of aspirin and ibuprofen on GI permeability during exercise. Int J Sports Med. 2007;28(9):722–726.
- Lambert GP, Broussard LJ, Mason BL, et al. Gastrointestinal permeability during exercise: effects of aspirin and energy-containing beverages. J Appl Physiol. 2001;90(6):2075–2080.
- Zuhl MN, Lanphere KR, Kravitz L, et al. Effects of oral glutamine supplementation on exercise-induced gastrointestinal permeability and tight junction protein expression. J Appl Physiol. 2014;116(2):183–191. (1985).
- Zuhl M, Dokladny K, Mermier C, et al. The effects of acute oral glutamine supplementation on exercise-induced gastrointestinal permeability and heat shock protein expression in peripheral blood mononuclear cells. Cell Stress Chaperones. 2015;20(1):85–93.
- Byrne C, Lee Jasonkaiwei, Chew Serenaaineo, et al. Continuous thermoregulatory responses to mass-participation distance running in heat. Med Sci Sports Exerc. 2006;38(5):803–810.
- Racinais S, Moussay S, Nichols D, et al. Core temperature up to 41.5 masculineC during the UCI road cycling world championships in the heat. Br J Sports Med. 2019;53(7):426–429.
- Hall DM, Buettner GR, Matthes RD, et al. Hyperthermia stimulates nitric oxide formation: electron paramagnetic resonance detection of .NO-heme in blood. J Appl Physiol. 1994;77(2):548–553. (1985).
- Costa RJS, Gaskell SK, McCubbin AJ, et al. Exertional-heat stress-associated gastrointestinal perturbations during Olympic sports: management strategies for athletes preparing and competing in the 2020 Tokyo Olympic Games. Temperature. 2020;7(1):58–88. doi: https://doi.org/10.1080/23328940.2019.1597676
- Ribeiro Hudson AS, Nascimento Soares AD, Coelho Horta NA, et al. The magnitude of physical exercise-induced hyperthermia is associated with changes in the intestinal permeability and expression of tight junction genes in rats. J Therm Biol. 2020;91:102610.
- Keirns BH, Koemel NA, Sciarrillo CM, et al. Exercise and intestinal permeability: another form of exercise-induced hormesis? Am J Physiol Gastrointest Liver Physiol. 2020;319(4):G512–G518.
- Bruchim Y, Horowitz M, Aroch I. Pathophysiology of heatstroke in dogs - revisited. Temperature. 2017;4(4):356–370. doi: https://doi.org/10.1080/23328940.2017.1367457
- Al Mahri S, Bouchama A. Heatstroke. Handb Clin Neurol. 2018;157:531–545.
- Kenny GP, Wilson TE, Flouris AD, et al. Heat exhaustion. Handb Clin Neurol. 2018;157:505–529.
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiol Rev. 1974;54(1):75–159.
- Burke S, Hanani M. The actions of hyperthermia on the autonomic nervous system: central and peripheral mechanisms and clinical implications. Auton Neurosci. 2012;168(1–2):4–13.
- Sharma HS, Westman J, Nyberg F. Chapter 18 pathophysiology of brain edema and cell changes following hyperthermic brain injury. In: Sharma, HS, Westman, J, editors. Brain function in hot environment. 1998. p. 351–412.
- Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol. 2014;4(4):1339–1368.
- DeLano FA, Hoyt DB, Schmid-Schönbein GW. Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock. Sci Transl Med. 2013;5(169):169ra11.
- Vargas N, Marino F. Heat stress, gastrointestinal permeability and interleukin-6 signaling - Implications for exercise performance and fatigue. Temperature. 2016;3(2):240–251. doi: https://doi.org/10.1080/23328940.2016.1179380
- Schmid-Schönbein GW. Analysis of inflammation. Annu Rev Biomed Eng. 2006;8:93–131.
- Park BS, Lee JO. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp Mol Med. 2013;45:e66.
- Waldo SW, Rosario HS, Penn AH, et al. Pancreatic digestive enzymes are potent generators of mediators for leukocyte activation and mortality. Shock. 2003;20(2):138–143.
- Chang M, Kistler EB, Schmid-Schönbein GW. Disruption of the mucosal barrier during gut ischemia allows entry of digestive enzymes into the intestinal wall. Shock. 2012;37(3):297–305.
- Pelaseyed T, Bergström JH, Gustafsson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev. 2014;260(1):8–20.
- Chang M, Alsaigh T, Kistler EB, et al. Breakdown of mucin as barrier to digestive enzymes in the ischemic rat small intestine. PLoS One. 2012;7(6):e40087.
- Altshuler AE, Penn AH, Yang JA, et al. Protease activity increases in plasma, peritoneal fluid, and vital organs after hemorrhagic shock in rats. PLoS One. 2012;7(3):e32672.
- Rosario HS, Waldo SW, Becker SA, et al. Pancreatic Trypsin Increases Matrix Metalloproteinase-9 Accumulation and Activation during Acute Intestinal Ischemia-Reperfusion in the Rat. Am J Pathol. 2004;164(5):1707–1716.
- Penn AH, Hugli TE, Schmid-Schönbein GW. Pancreatic enzymes generate cytotoxic mediators in the intestine. Shock. 2007;27(3):296–304.
- Penn AH, Schmid-Schönbein GW. The intestine as source of cytotoxic mediators in shock: free fatty acids and degradation of lipid-binding proteins. Am J Physiol Heart Circ Physiol. 2008;294(4):H1779–92.
- Santamaria MH, Aletti F, Li JB, et al. Enteral tranexamic acid attenuates vasopressor resistance and changes in alpha1-adrenergic receptor expression in hemorrhagic shock. J Trauma Acute Care Surg. 2017;83(2):263–270.
- Altshuler AE, Lamadrid I, Li D, et al. Transmural intestinal wall permeability in severe ischemia after enteral protease inhibition. PLoS One. 2014;9(5):e96655.
- DeLano FA, Schmid-Schönbein GW. Pancreatic digestive enzyme blockade in the small intestine prevents insulin resistance in hemorrhagic shock. Shock. 2014;41(1):55–61.
- Bauza-Martinez J, Aletti F, Pinto BB, et al. Proteolysis in septic shock patients: plasma peptidomic patterns are associated with mortality. Br J Anaesth. 2018;121(5):1065–1074.
- Moseley PL, Gapen C, Wallen ES, et al. Thermal stress induces epithelial permeability. Am J Physiol Cell Physiol. 1994;267(2):C425–C434.
- Fitzal F, DeLano FA, Young C, et al. Pancreatic enzymes sustain systemic inflammation after an initial endotoxin challenge. Surgery. 2003;134(3):446–456.
- Hosokawa Y, Casa DJ, Trtanj JM, et al. Activity modification in heat: critical assessment of guidelines across athletic, occupational, and military settings in the USA. Int J Biometeorol. 2019;63(3):405–427.
- World Health Organization, World report on ageing and health. World Health Organization, 2015.
- Lefkowitz RB, Schmid-Schönbein GW, Heller MJ. Whole blood assay for trypsin activity using polyanionic focusing gel electrophoresis. Electrophoresis. 2010;31(14):2442–2451.




