ABSTRACT
While it is clear that the ovarian hormones estradiol and progesterone have important influences on physiological thermoregulation in women, the influences of these hormones on responses to cold exposure are not well understood. Both heat conservation and heat production must increase to offset heat losses that decrease body temperature in cold ambient conditions. Cutaneous vasoconstriction conserves heat, whereas shivering and non-shivering thermogenesis produce heat – all as part of reflex physiological responses to cold exposure. Our goal in this brief review is to highlight existing knowledge and recent advances pertaining to sex and sex hormone influences on thermoeffector responses to cold stress. Estrogens have multiple influences that contribute to heat dissipation and a lower body temperature, while the influence of progesterone appears to primarily increase body temperature. Fluctuations in estrogen and progesterone across the menstrual cycle can alter the level at which body temperature is regulated. Recent evidence suggests that female reproductive hormones can modulate the cutaneous vasoconstrictor response, and may influence metabolic mechanisms such as substrate utilization during shivering and non-shivering thermogenesis. Overall, it appears that quantitative differences in cold thermoregulation between sexes are minimal when anthropometric measures are minimized, such that women do not have a strong “advantage” or “disadvantage” in terms of overall ability to tolerate cold. Thermoregulatory physiology in women during cold exposure remains relatively understudied and many mechanisms require further elucidation.
INTRODUCTION
The female reproductive hormones estradiol and progesterone have distinct effects on regulation of body temperature. These have been recognized, but incompletely understood, for several decades. For example, core body temperature increases by ~0.5°C in the luteal phase of the menstrual cycle (likely due to elevated progesterone) [Citation1,Citation2], reflecting a shift in thermoregulatory control toward this higher temperature. Estradiol, on the other hand, tends to promote heat dissipation, both via direct effects on peripheral vasodilation and via influences on central autonomic nuclei controlling the balance point (i.e., the level at which body temperature is regulated) and thermoeffector responses [Citation3–6].
The influence of female reproductive hormones on thermoregulatory responses to cold exposure is a relatively understudied area. From an applied perspective for athletes, the military and others, women may have “advantages” (e.g., glycogen sparing during shivering) and/or “disadvantages” (e.g., greater surface area-to-mass ratio) when it comes to regulating their body temperature during exposure to cold environments. The goal of the present brief review is to summarize existing knowledge regarding physiological thermoregulation in the cold in women, with a focus on the influences of female reproductive hormones estradiol and progesterone.
The regulation of body temperature is influenced by both physiological (e.g., autonomic) and behavioral (e.g., huddling, donning warm clothing, engaging in voluntary activity) responses. Thermal behavior is mediated by changes in thermal perception and is driven by the perception of thermal discomfort [Citation7–9]. Perceptual responses to thermal stress differ between sexes [Citation10–12]. It is often reported that women experience greater thermal discomfort in cold environments [Citation13,Citation14], which suggests that women may use behavioral thermoregulation to a greater extent in the cold than men. Further, there is evidence that female reproductive hormones can influence thermal perception in the cold and that thermal behavior may vary across the menstrual cycle in women [Citation15,Citation16]. However, few studies have been conducted to mechanistically examine the influence of sex and reproductive hormones on the activation of behavioral thermoeffector responses in cold-exposed humans. Thus, the primary focus of this review is the influence of female reproductive hormones on peripheral and central autonomic thermoeffector responses, though sex and menstrual cycle differences in behavioral thermoregulation and the interaction between autonomic and behavioral thermoeffector responses in the cold remain important topics of investigation for future work. For interested readers, an up-to-date understanding of human thermal behavior in cold environments has been expertly reviewed elsewhere [Citation9].
OVERVIEW OF HUMAN PHYSIOLOGICAL RESPONSES TO COLD
Cutaneous Vasoconstriction
In response to body cooling, afferent signals from peripheral and central thermoreceptors are sensed by the pre-optic region of the anterior hypothalamus (PO/AH), from which efferent signals elicit insulative (i.e., cutaneous vasoconstriction) and metabolic (e.g., shivering and nonshivering thermogenesis) thermoregulatory effector responses. The early and sustained response to cold exposure is characterized by constriction of cutaneous blood vessels and a subsequent reduction in skin blood flow [Citation17,Citation18]. The vasoconstrictor response to skin cooling reduces the skin-to-air temperature gradient and thus minimizes convective heat loss during cold exposure. As cooling continues and cutaneous vasoconstriction becomes insufficient to prevent decreases in core temperature, metabolic heat production increases to offset heat loss [Citation19,Citation20].
During environmental cooling, cutaneous vasoconstriction has two components: a peripherally mediated local response and a centrally mediated reflex response elicited by cooling over a larger body surface area [Citation21,Citation22]. Local and reflex cooling mechanisms act together to determine the total vasoconstrictor response to a given ambient cold exposure. Reflex control of cutaneous vasomotor tone is mediated by the sympathetic nervous system (SNS) and downstream adrenergic [i.e., norepinephrine (NE)] and nonadrenergic mechanisms [Citation23]. Increases in cutaneous sympathetic nerve activity during cold exposure stimulates the release of NE which subsequently binds to and activates α-receptors on the vascular smooth muscle. In young healthy individuals, NE mediates approximately 60% of the total reflex vasoconstrictor response. Additional neurotransmitters are co-released with NE from sympathetic nerve endings and are responsible for the remaining ~40% of the response [Citation23,Citation24]. The co-transmitters involved most likely include neuropeptide Y (NPY) and ATP, identified via intradermal application of Y1- and P2x-receptor antagonists, respectively [Citation25,Citation26]. Within the cutaneous vascular smooth muscle, multiple intracellular second-messengers play a role in reflex vasoconstriction, the most notable being the Rho-kinase (ROCK) pathway [Citation27]. ROCK is an important pro-vasoconstrictor mediator that stimulates vascular smooth muscle contraction by 1) inhibiting myosin light-chain phosphatase and thus causing calcium sensitization and 2) inducing the translocation of α2C-receptors to the cell membrane from the Golgi apparatus [Citation28]. ROCK-signaling contributes to reflex vasoconstriction during whole-body cooling in young healthy adults; furthermore, the reliance on ROCK increases greatly when other adrenergic mechanisms are compromised (e.g., with aging or clinical disease) [Citation27].
In addition to central reflex mechanisms, cutaneous vasoconstriction is mediated by local mechanisms (i.e., the direct effects of local temperature on the neuroeffector junction and blood vessels themselves). These mechanisms also include adrenergic and nonadrenergic components [Citation29,Citation30]. The initial local vasoconstrictor response is primarily mediated by the translocation of intracellular α2C-receptors to the smooth muscle cell membrane and subsequent activation by NE [Citation31]. Alpha2C-receptor translocation during local cooling is stimulated by reactive oxygen species (ROS)-induced activation of ROCK [Citation28,Citation32–34]. As skin temperature continues to decline, local mechanisms suppress the nitric oxide (NO) system by inhibiting NO synthase and downstream NO-dependent signaling, further enhancing the vasoconstrictor response during prolonged cooling [Citation29].
Shivering Thermogenesis
Following the early cutaneous vasoconstrictor response, humans must rely on an increase in metabolic rate to guard against excessive decreases in body temperature. In contrast to the capacity for heat dissipation, the capacity for heat production in humans is limited. For example, if a sweat rate of 2.5 L/h and complete evaporation of sweat is assumed, a theoretical value of 1700 W of heat loss could be passively achieved [Citation35,Citation36]; whereas the highest recorded metabolic heat production in shivering humans during cold water immersion is ~760 W [Citation37]. However, in instances where survival may be imperative, initiation of whole-body exercise can generate metabolic heat exceeding 1,000 W in cold conditions [Citation38,Citation39]. Although the rate at which metabolic heat can be produced via exercise should be appreciated, exercise cannot be sustained indefinitely, thus shivering remains a valuable heat producing thermoeffector.
Heat production is accomplished through shivering (ST) and non-shivering thermogenesis (NST), with the former being the primary contributor to total thermogenic activity in humans. Shivering thermogenesis is achieved by involuntary, rhythmic, repetitive contraction of skeletal muscle whereby the majority of metabolic energy is liberated as heat. Shivering generally starts in the torso muscles and propagates toward the limbs [Citation40]. As shivering intensifies, more muscles are recruited, and whole-body metabolic rate consequently increases. Maximally, ST at rest can increase resting metabolic rate (RMR) ~5.0 fold in uncompensable cold conditions [Citation37].
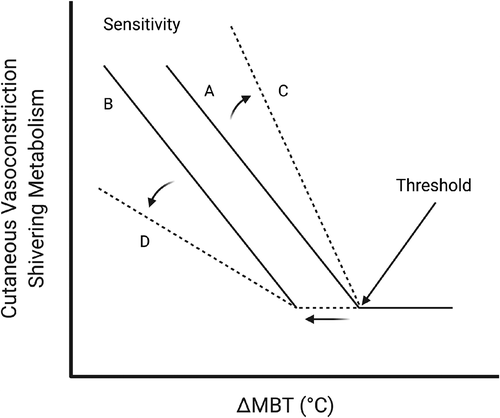
NST is heat production occurring primarily in brown adipose tissue (BAT) and skeletal muscle that is not associated with shivering muscle contraction (but not separate from skeletal muscle contraction entirely; see Non-Shivering Thermogenesis in Skeletal Muscle) [Citation41,Citation42]. Shivering skeletal muscle is innervated by alpha and gamma motoneurons that activate upon efferent signaling from peripherally and centrally integrated thermoreceptors [Citation43,Citation44]. Shivering onset occurs first in response to reductions in skin temperature and shivering intensity increases with cold stress severity as core temperature declines. There is a feed-forward aspect to this response, which primes heat-producing thermoeffectors (e.g. ST and NST) to activate during decreases in surface temperature in anticipation of core temperature decreases, such that excessive declines in core temperature do not readily occur when imposed by cold stress. The regulation of mean body temperature (integration of skin and core temperature) dictates the effector response. In cold, as mean body temperature (MBT) declines, a thermoeffector response is elicited upon reaching a sufficient ∆MBT (threshold) (). The degree of the effector response, referred to as sensitivity, is represented by the slope of the relationship to threshold onset. Therefore, shifts in threshold or perturbations in sensitivity can enhance or attenuate the effector response [Citation45,Citation46].
Figure 1. Schematic representation of the thermal effector output-to-change in mean body temperature (ΔMBT) relationship during cold exposure. As mean body temperature decreases, an inflection point is reached, known as the threshold, at which heat-conserving (cutaneous vasoconstriction) or heat-generating (shivering) responses begin to increase (line A). The slope of the thermoeffector output-to-ΔMBT relationship represents the sensitivity of the response. Lines B-D are examples of how the effector-ΔMBT relationship can change. Lines B and D demonstrate a leftward shift in threshold, such that a larger ΔMBT is required to elicit the thermoeffector response. Lines C and D represent an increase or decrease in sensitivity, respectively, such that a larger or smaller change in thermoeffector output occurs for a given ΔMBT. Adapted from Castellani and Young (2016) with permission. Figure was created with BioRender.com.
Shivering skeletal muscle can dynamically shift substrate utilization, a phenomenon that is dependent on cold stress severity [Citation47]. As skin and/or core temperature decline with continued cold exposure, metabolic rate increases, thus the relative contribution of carbohydrates (CHO) increase, while lipids decrease, as shivering intensifies [Citation48,Citation49]. At shivering intensities below 50% of maximal shivering intensity, lipids are the predominant fuel source with CHO and protein oxidation contributing to a lesser degree [Citation48,Citation49]. At shivering intensities above 50% of maximal shivering intensity, reliance on CHO oxidation increases, and dependence on lipids and proteins subsequently decrease [Citation48,Citation49]. In contrast to exercise, where the contribution of protein is negligible, protein can account for ~20% of fuel metabolism during mild shivering in a glycogen depleted state [Citation50,Citation51]. Thus, shivering skeletal muscle substrate selection is plastic and can modulate its fuel mixture based on available substrates to maintain heat production. The interested reader is directed to the reviews of Haman and Blondin for further knowledge on shivering muscle substrate utilization [Citation20,Citation52].
Although BAT contributes to heat production in adult humans, there is some debate about the extent to which it contributes to total heat production during cold exposure. However, the contribution of BAT and NST cannot be discounted as the recruitment of BAT or inhibition of BAT metabolism can delay or accelerate shivering onset and intensity [Citation53,Citation54]; indicating an orchestrated effort between shivering and nonshivering thermogenesis in heat production and core temperature maintenance during cold exposure.
BAT Thermogenesis
The existence and functional nature of BAT has long captivated the scientific community since its discovery in the 16th century [Citation55]. Remarkably, this brown, multilocular tissue has inspired multiple resurgences in concerted research efforts for over four centuries. Early observations in hibernating rodents found BAT to be highly thermogenic, defending against decreases in core temperature and serving as a mechanism for energy homeostasis [Citation56]. Given the significantly larger body surface area (BSA)-to-mass ratio of rodents relative to humans, it could be speculated a greater reliance on BAT thermogenesis would be expected and beneficial to survival. While abundant in small mammals, BAT in humans was thought to exist only in newborns, with atrophy occurring throughout infancy and minimal levels soon after [Citation57]. This diminution of BAT raised speculation that its purpose was physiologically inconsequential in adult humans. Nonetheless, the potential for anti-obesity therapeutics (e.g., potential for burning extra calories without exercise) remained a significant and perpetuating impetus for the continuation of BAT research in rodents [Citation58].
The quantitative contribution of BAT thermogenesis in humans remains a contentious topic in cold research. In 1961, Davis et al. first demonstrated the capacity for induced cold acclimation in men [Citation59]. It was concluded that an attenuation of shivering intensity was a hallmark adaptation to cold and was likely attributable to enhanced NST, analogous to observations in cold adapted rodents [Citation59]. However, reanalysis of these data over five decades later suggested Davis’ report may not to be definitive evidence of BAT-mediated NST [Citation45]. In 1981, the presence of human adult BAT was described from autopsied adipose samples in Finnish outdoor workers [Citation60]. In contrast to indoor workers, the occurrence of BAT was greater in occupations where individuals chronically performed work in cold conditions. While these BAT depots were indeed histochemically divergent from white adipose tissue, in vivo confirmation of metabolic activity remained undetermined. In 2009, utilizing 18F-fluorodeoxyglucose (18F-FDG) tracing and combination positron emission computed tomography (PET/CT) techniques, large cold-activated BAT depots were identified in adult humans [Citation61–64] and were confirmed to be metabolically active [Citation65]; yet again prompting a surge in research interest, which has persisted over the past decade.
Unique to brown adipocytes is the expression of mitochondrial uncoupling protein (UCP) 1 which confers its thermogenic effect [Citation66,Citation67]. This phenomenon is accomplished through an increased dissipation of the electrochemical proton gradient across the inner mitochondrial membrane to be liberated as heat, rather than to power ATP synthesis. This uncouples oxygen consumption from ATP synthesis [Citation67]. Further adding to the intrigue surrounding BAT is the “browning” effect, in which white adipocytes inherit thermogenic properties similar to classical brown adipocytes. These heat-generating white adipocytes are referred to as “beige” or “brite” (brown-in-white) adipocytes [Citation68].
BAT is characterized by rich sympathetic innervation, high vascularization, and exceptionally high mitochondrial content [Citation69,Citation70]. A high level of vascularization serves to deliver circulating energy substrates for BAT metabolism and to carry NST-generated heat into the systemic circulation. Efferent sympathetic nerve fibers innervate BAT and SNS outflow is the ultimate activator of BAT thermogenesis [Citation70,Citation71]. SNS-mediated release of NE binds to β-adrenergic receptors (β-AR) present on the individual brown adipocyte cell surface and tissue vasculature. It has long been believed that β3-AR induces BAT thermogenesis in humans, as it does in rodents [Citation64,Citation72,Citation73]. However, new evidence proposes that BAT in humans also utilize β1-AR and β2-AR, to stimulate BAT, rather than β3-AR [Citation74,Citation75]. NE binding initiates the adenylyl cyclase/cAMP/protein kinase A signaling cascade which, subsequently, results in hydrolysis of intracellular triglycerides [Citation66,Citation76]. These newly mobilized long chain fatty acids resultant of triglyceride hydrolysis serve two functions: 1) the activation of the UCP1 and initiation of UCP1 mediated thermogenesis and 2) provide necessary fuel for BAT thermogenesis. Thus, BAT intracellular triglycerides act as a vital mediator in BAT thermogenesis and the primary substrate for BAT metabolism. The interested reader seeking further information on UCP1 function, BAT metabolism, and neural control is referred to the excellent reviews of Richard et al. [Citation77], Carpentier et al. [Citation78], and Morrison et al [Citation79].
In adult humans, BAT depots are most abundant in the supraclavicular region, followed by the thoracic, paraspinal, and perirenal regions [Citation61,Citation63,Citation80]. BAT prevalence and volume is dependent on age, body fat, sex, outdoor temperature, and cold acclimation status. BAT volume and activity decreases with age [Citation61–63,Citation80,Citation81] and is correlated with anthropometric factors. Indeed, detectable BAT volume and activity is higher in individuals with lower BMI and lean body mass, diminishes with increased percent body fat, and is minimally detectable in obese individuals [Citation61–63,Citation81]. It is generally understood from retrospective studies that women possess more BAT than men [Citation61,Citation80,Citation82,Citation83Citation83]; although contradictory evidence exists [Citation84,Citation85]. However, many of these trends are established from retrospective epidemiological data in patient populations that implemented “fixed” cooling protocols (e.g., identical cold stress across all participants) and did not aim to control cold acclimation status [Citation86]. Women perceive identical cold stress to be cooler than men and the thermoneutral zone – the range of ambient temperatures that do not induce changes in metabolic heat production or evaporative heat loss – is suggested to be higher in women than men [Citation11,Citation13,Citation87]. These factors could underlie the higher incidence of reported BAT prevalence, volume, and activity in women if the ambient temperature is low enough to stimulate BAT thermogenesis in women but not men. Therefore, many prospective studies have demonstrated that these relationships are not casual or consistently reproducible in controlled environments.
BAT prevalence is influenced by outdoor temperature and exhibits seasonal variation. BAT is most readily detectable in the winter months when ambient temperatures are lowest, with prevalence decreasing in the fall, spring, and summer months, respectively, as ambient temperatures rise [Citation61,Citation62,Citation80]. Multiple studies have demonstrated increased BAT recruitment following repeated cold exposure. BAT prevalence, volume and activity can be substantially increased following cold acclimation in both men and women [Citation85,Citation88–91], though there does not appear to be any difference in magnitude of recruited BAT based on sex. Ten days of cold acclimation at ~15°C analogously increased BAT volume and activity in men and women [Citation85]. Blondin et al. reported a 45% and 182% increase in BAT volume and oxidative metabolism, respectively, after a 4-week cold acclimation regimen [Citation88]. Interestingly, Yoneshiro et al. described the recruitment of BAT in previously BAT negative individuals (i.e., those who had undetectable BAT activity during acute cold exposure) following cold acclimation [Citation91].
Non-Shivering Thermogenesis in Skeletal Muscle
NST in humans is not exclusively reserved to BAT thermogenesis. While BAT has received significant research attention over the past decades, skeletal muscle-derived NST in humans is an emerging and relevant topic. The capacity for cold-induced thermogenesis in non-shivering skeletal muscle has been historically observed in vertebrates that lack BAT, namely various avian species and mammals [Citation92]. Similar to the research arc of BAT, muscle-derived NST has been viewed as a potential target for novel antiobesity therapeutics, and little is known in humans [Citation42].
The physiological mechanisms which enable the thermogenic effect of skeletal muscle NST remain unclear. One mechanism that has been described more completely in small mammals, but understood incompletely in humans, is sarcoendoplasmic reticulum Ca2+ ATPase (SERCA) pump activity. In this mechanism, heat is liberated via ATP hydrolysis when facilitating Ca2+ transport between cell cytoplasm and the sarcoplasmic reticulum lumen (see [Citation93,Citation94] for detailed reviews of SERCA function and modifiers). Indeed, an action potential is required for the release of Ca2+, which may concomitantly induce muscle contraction and futile Ca2+ cycling. Thus, these events are viewed as independent, but are intrinsically associated with skeletal muscle activation.
Another proposed mechanism is mitochondrial uncoupling, similar to adrenergically mediated “proton leaking” occurring in BAT thermogenesis; although this uncoupling occurs in a non-UCP1 dependent manner. Rather, UCP3 activity sensitized via ROS upregulation may facilitate proton leak mechanics [Citation95]. Indeed, adaptive thermogenesis was first observed following calorie restriction in overweight women, which demonstrated mitochondrial proton leak and upregulated UCP3 mRNA, and was responsible for increased energy expenditure (EE) [Citation96].
In support, limited data from mild cold exposure studies suggest mitochondrial proton leak to be a likely mechanism. In 2008, Wejiers et al. documented the capacity for uncoupled mitochondrial respiration (state 4 respiration) in non-shivering skeletal muscle during an acute 22°C cold exposure in young men [Citation97]. Later, Wejiers et al. importantly demonstrated that during β1-AR and β2-AR blockade, cold-induced increases in EE persisted [Citation98]. However, cold thermogenesis was no longer associated with state 4 respiration in skeletal muscle tissue. It was therefore concluded that muscle-derived NST is responsible for cold thermogenesis when β-receptors are functionally intact but that a non-β1 and β2 mechanism, potentially β3-mediated mitochondrial uncoupling in BAT, compensates when β2 receptors, the predominant receptor in skeletal muscle, are blocked [Citation98].
Over the past few years, few studies have aimed to evaluate the metabolic contribution of skeletal muscle to cold-induced thermogenesis in humans. Din et al. selectively measured BAT oxygen consumption using [15O]O2 PET imaging in men and women, and determined that BAT contributed only ~1% to whole-body elevations in EE following a mild cold stimulus; whereas skeletal muscle thermogenesis contributed ~40% to changes in cold-induced EE [Citation99]. Interestingly, it has been shown that muscle NST is responsive to cold acclimation, which appears to cause a shift toward BAT thermogenesis without a net change in whole-body thermogenesis. Blondin et al. implemented a 4-week acclimation regimen which resulted in reduced skeletal muscle proton leak and EMG-measured shivering activity [Citation88]. Moreover, these decrements were accompanied by significant increases in BAT volume and metabolism [Citation88].
Little is known about cold-induced proton leak in human skeletal muscle. Future research is certainly warranted given that humans possess substantially greater muscle mass than BAT mass (42% vs <1% of total body mass) and skeletal muscle contributes significantly more to whole-body thermogenesis than BAT (~40% vs ~1%) [Citation86,Citation99,Citation100]. Moreover, the primary mechanisms from which metabolic heat production occur require further elucidation. While UCP3 activity seems to be modulated by ROS to some degree [Citation95], cold exposure, whether acute or chronic, does not seem to elevate UCP3 levels, leaving some uncertainty to its ultimate influence in a cold-adaptive response [Citation88,Citation97]. Relevant to the current review, muscle-derived NST remains uninvestigated in women. Therefore, future research should aim to include women and evaluate the effect of female sex hormones on the skeletal muscle NST response to cold exposure, particularly with regard to menstrual cycle influences in NST recruitment.
OVERVIEW OF FEMALE REPRODUCTIVE HORMONES
Reproductive Hormones in Young and Older Women
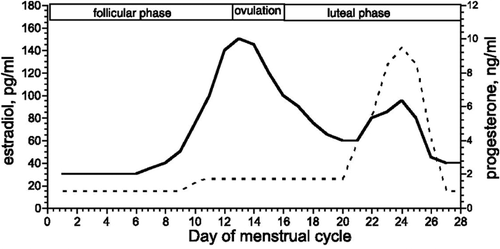
In young healthy women, the reproductive hormones estradiol and progesterone increase and decrease over the course of the menstrual cycle, which lasts approximately 28 days [Citation101–103]. The cycle is considered to start on the first day of menstruation, which is usually counted as day 0 or 1 of the cycle. Around day 10, there is an increase in circulating estradiol leading to ovulation around day 14. After ovulation, the level of circulating estradiol decreases rapidly, followed by a smaller increase that coincides with increasing levels of progesterone during the luteal phase. Progesterone concentration peaks during the mid-luteal phase (around day 21), after which both hormones decrease back to the early follicular levels and the cycle begins again with menstruation. The result of these regular fluctuations is that both estradiol and progesterone are at their lowest of the cycle during the early follicular phase. Estradiol is at its highest level and “unopposed” by progesterone for a brief period in the late follicular/ pre-ovulatory phase, and both progesterone and estradiol are elevated during the mid-luteal phase (see for schematic overview [Citation104,Citation105,Citation106]).
Figure 2. Typical concentrations of plasma estradiol and progesterone over a normal menstrual cycle. Circulating concentrations of estradiol (solid line) and progesterone (dashed line) are lowest during the early follicular phase (first 5–7 days of the cycle). Leading up to ovulation (around days 10–14), circulating estradiol increases to its highest level. After ovulation, there is a significant decrease in estradiol, followed by a smaller increase that coincides with a large rise in progesterone. Progesterone and estradiol concentrations are elevated during the mid-luteal phase (around days 21–24), after which both hormones decline and the cycle begins again with the next menses. From Stachenfeld and Taylor (2009). Reproduced with permission.
During oral contraceptive use, endogenous cycling of estradiol and progesterone is suppressed [Citation103,Citation104] . Depending on the type of contraceptive used, there is usually a 3-week period during which the exogenous hormones are elevated in the circulation, followed by a one-week period (the placebo or no-pill week) during which hormone levels decrease. Some young women have months during which they have anovulatory cycles even though they are not taking oral contraceptives. Reasons for these in otherwise healthy women include high levels of physical (athletic) or emotional stress, low caloric intake, low BMI and others [Citation107,Citation108]. In these women the normal monthly fluctuations in estradiol and progesterone may be diminished or altogether absent.
The perimenopausal period (usually around late 40s to early 50s) is associated with less regular fluctuations in reproductive hormones, more irregularity of menstrual cycles. After definitive menopause (defined as ~2 years without a regular menstrual period), women experience dramatic decreases in the overall circulating levels of estradiol and progesterone. In some research studies, the comparison of responses between pre- and post-menopausal women has provided important insight into the potential roles of female reproductive hormones (particularly estradiol) in physiological mechanisms [Citation104,Citation109].
Thermoregulatory Effects of Estradiol and Progesterone
In young women, the mid-luteal phase of the menstrual cycle is associated with an increase in core body temperature of ~0.5°C, when compared to the early follicular phase [Citation104,Citation110–112]. This is a regulated increase in temperature, as shown by the shifts in thermoregulatory control of shivering, sweating and skin blood flow toward the defense of this higher temperature [Citation110,Citation111,Citation113]. However, the regulated increase in temperature does not occur by the same mechanisms as those seen in an infection-induced fever, since they are not reversed by cyclooxygenase inhibition [Citation114]. The luteal phase shift appears to be primarily an effect of progesterone, since when estradiol is “unopposed” by progesterone, such as in the pre-ovulatory phase of the menstrual cycle, body temperature is regulated at lower levels [Citation6].
There is some evidence from neural recordings of the PO/AH region that estradiol and progesterone have direct influences on temperature-sensitive neurons, which likely mediate part of their thermoregulatory effects [Citation5,Citation115]. Interestingly, progesterone and estradiol seem to act in opposition to one another – although when both hormones are elevated (as in the luteal phase or with combination oral contraceptives), the thermogenic effect of progesterone is usually predominant [Citation113].
At rest in thermoneutral temperatures, the major thermoregulatory effector in humans is the sympathetic noradrenergic vasoconstrictor system in the skin [Citation116,Citation117]. That is, during most daily activities, core body temperature does not change much and the responses needed to maintain thermal equilibrium are achieved almost exclusively through small changes in the level of skin blood flow via increases and decreases in the activity of cutaneous vasoconstrictor nerves [Citation117]. This raises an interesting question regarding resting, thermoneutral values for skin blood flow and/or metabolic rate in the luteal phase compared to the follicular phase of the menstrual cycle (or for other comparisons of high vs low progesterone). It would make sense that skin blood flow would be lower, and/or resting metabolic rate higher, in resting/ thermoneutral conditions in the luteal phase (due to the basic biophysics of heat balance). One or the other (or both) of these possibilities may be the case. However, the amount that resting skin blood flow or metabolism would need to change to support this small increase in temperature would be so small (< 2%) as to be below the resolution of available measurement techniques [Citation117–119] – so this remains an open question.
SEX AND REPRODUCTIVE HORMONE DIFFERENCES IN THE VASOCONSTRICTOR RESPONSE TO COLD
Cutaneous Vasoconstriction in Non-Acral Skin
As noted above, the observed increase in resting body temperature in the luteal phase of the menstrual cycle [Citation104,Citation110–112] has raised questions surrounding the influence of female reproductive hormones on vasoconstrictor activity in the skin microcirculation. Estrogen and progesterone modulate sympathetic activity [Citation120,Citation121] and adrenergic receptor and co-transmitter expression and sensitivity [Citation122–125]. Estrogen receptors are present on the endothelium and vascular smooth muscle, and there is ample evidence that estrogen and progesterone can alter peripheral vascular responsiveness [Citation126–131]. Therefore, it is plausible that female reproductive hormones may modulate cutaneous vasoconstriction across the menstrual cycle in women. In an effort to examine potential alterations in reflex control of the cutaneous vasoconstrictor system, Charkoudian and Johnson measured skin blood flow during whole-body cooling in women taking oral contraceptives [Citation132]. Similar to the shift in cutaneous vasodilation to higher body temperatures during heat stress, the control of the vasoconstrictor response during cooling was reset around a higher body temperature in the high hormone phase of oral contraceptive use. However, the sensitivity of the cutaneous vasoconstrictor response during whole-body cooling was unaffected by hormone status. In support of this notion, no change in end-organ (i.e., microvessel) responsiveness to local forearm skin cooling was found between low and high reproductive hormone status [Citation133].
Interestingly, while there is no difference in the magnitude of the vasoconstrictor response, the contributions of NE and nonadrenergic co-transmitter-mediated reflex vasoconstriction during whole-body cooling appear to be altered by hormone status during oral contraceptive use. Using intradermal injection of yohimbine and propranolol to block the action of NE on adrenergic receptors, Stephens et al. demonstrated that nonadrenergic mechanisms contribute to reflex cutaneous vasoconstriction in the high hormone phase, but not the low hormone phase [Citation134]. This finding is consistent with the ability of female reproductive hormones to alter gene expression of sympathetic co-transmitters and their receptors. Specific to reflex vasoconstriction, progesterone has been suggested to increase hypothalamic NPY expression and modify Y1 and Y2 receptor expression following estrogen pre-treatment [Citation124,Citation125,Citation135]. Surprisingly, the contribution of nonadrenergic mediators to the reflex response in the high hormone phase in women matches a similar response to that in men [Citation23], though the reason(s) for this finding remains unclear. While older generation progestins in the 1960s have been known to have androgenic effects [Citation136,Citation137], the authors noted that the oral contraceptives used in this study were not likely to have the same level of androgenicity as previous synthetic progestins and, though not measured in this study, combined oral contraceptives have even been associated with reduced circulating testosterone concentrations [Citation138–140]. However, synthetic female ovarian hormones may have nonspecific effects on mediators of reflex vasoconstriction that could contribute to this unexpected finding. The influence of both exogenous and endogenous reproductive hormones on cutaneous vasoconstrictor signaling pathways during environmental cooling requires further investigation.
Cutaneous Vasoconstriction in Acral Skin
In contrast to non-acral, or hairy, skin, acral skin (e.g., palm of the hand and sole of the foot) is richly innervated by sympathetic vasoconstrictor nerves and contains arteriovenous anastomoses, direct low-resistance connections between arterioles and venules that can cause considerable changes in skin blood flow [Citation141–143]. The influence of sex and female reproductive hormones on local vasoconstrictor responses in cold-exposed acral skin has been an important topic of investigation. This area of research has clear clinical relevance, as women are much more likely to suffer from Raynaud’s phenomenon, a vasospastic syndrome characterized by excessive skin vasoconstriction in the extremities [Citation144,Citation145]. Furthermore, vasospastic symptoms are known to improve after menopause, but not in post-menopausal women taking estrogen replacement therapy, which supports a role for female reproductive hormones in the exaggerated vasoconstrictor response in acral skin [Citation146,Citation147]. In line with these epidemiological observations, controlled laboratory investigations demonstrate that local control of hand cutaneous blood flow differs between sexes and across the menstrual cycle, which may explain in part the higher incidence of Raynaud’s in women.
A series of studies by Cankar and colleagues demonstrated that hand cooling elicits a substantially greater reduction in local cutaneous blood flow in women than in men [Citation148–150]. This exaggerated vasoconstrictor response is particularly pronounced in women taking oral contraceptives, followed by premenopausal women and postmenopausal women [Citation151]. Interestingly, the marked increase in vasoconstriction is not only observed at the direct site of cooling, but also at a remote or indirect skin site (i.e., finger of the contralateral hand), suggesting that a greater sympathetic reflex vasomotor response to hand cooling may occur in women [Citation149]. This finding is supported by greater indirect indices of sympathetic reactivity to hand cooling in women and greater parasympathetic reactivity in men [Citation148].
Across the menstrual cycle, the direct vasoconstrictor response to local hand cooling is greater in the luteal phase compared to the follicular phase [Citation149,Citation152], and microvascular responsiveness to cooling correlates with combined estrogen and progesterone concentrations in the follicular phase and progesterone concentrations in the luteal phase [Citation152]. In contrast to the difference between sexes, menstrual cycle phase differences are not observed in the remote response to local cooling. Thus, differences in reflex sympathetic activity (mediated through α1-adrenergic receptors) likely contribute to the sex differences in the response to digit cooling [Citation152], while changes across the menstrual cycle are likely to result from differences in local vascular mechanisms (i.e., changes in α-adrenergic receptor sensitivity and/or endothelium-derived vasomotor substances).
As indicated above, a significant portion of the local vasoconstrictor response to skin cooling is mediated by the translocation and subsequent activation of α2C-adrenergic receptors to the plasma membrane. In acral skin, women appear to rely more heavily on α2-adrenergic receptors during local cooling compared with men. This increased reliance on α2-adrenergic receptors is supported by the observation that the local vasoconstrictor response is abolished with α2-receptor blockade in women but not men [Citation150]. Estrogen is a potent activator of α2C-adrenergic receptor expression in vascular smooth muscle cells [Citation126]. In isolated human skin cells, 17β-estradiol augments the expression of α2C-adrenergic receptors in the cutaneous vascular smooth muscle, likely through actions on α and/or β estrogen receptors on the plasma membrane, and augments the cold-induced increase in α2-adrenergic receptor-mediated vasoconstriction [Citation126]. Taken together, α2-adrenergic receptors appear to have an important role in mediating sex and menstrual cycle differences in acral skin vasoconstriction at the local site of cooling and may be a critical mechanism underlying the increased prevalence of cold-induced vasospastic symptoms in women.
Following the initial sympathetically mediated vasoconstrictor response, acral skin also possesses the ability to increase blood flow through a phenomenon called cold-induced vasodilation or “the hunting reaction” [Citation141]. Cold-induced vasodilation is characterized by oscillatory periods of vasodilation and subsequent fluctuations of local skin temperature. Though the mechanisms remain largely unknown, there is no clear evidence to suggest that sex modulates the onset or frequency of the cold-induced vasodilation response [Citation141,Citation153]. However, future studies examining the potential influence of the menstrual cycle and reproductive hormone status on cold-induced vasodilation are warranted.
SEX AND REPRODUCTIVE HORMONE INFLUENCES ON SHIVERING THEROMEGENSIS
Shivering Onset and Activity
The rise in resting core temperature during the midluteal phase of the menstrual cycle is a defining thermoregulatory response to elevated progesterone concentrations. As demonstrated under heat stress, it is known this offset in core temperature during the luteal phase alters heat dissipation mechanisms [Citation110–114]. In cold, a similar phenomenon occurs in regard to shivering onset and heat production [Citation46,Citation110]. In the classical study of Hessemer et al., it was demonstrated that, parallel to threshold shifts for sweating and cutaneous vasodilation, the threshold temperature for shivering (assessed via EMG) was increased during the luteal phase, suggesting a shift in the thermoregulatory balance point [Citation110]. Over a decade later, Gonzalez et al. demonstrated that women exposed to cold during the luteal phase exhibited a lowered shivering sensitivity, using oxygen consumption as a proxy for shivering thermogenesis, which resulted in an attenuated MBT-metabolic heat production slope [Citation46]. The authors proposed this alteration in the shivering response was attributable to a readjustment in total heat balance [Citation46].
Contrary to the above studies, Blondin et al. found no difference in shivering onset or intensity across the menstrual cycle [Citation154]. In this investigation, skin and core temperature were clamped, and EMG-determined shivering onset occurred at similar body temperatures during the follicular and luteal phase [Citation154]. The reason for these discrepancies are unclear but may be due to methodological differences in cooling protocols among studies. Hessemer et al. and Gonzalez et al. utilized cold air exposures, whereas Blondin et al. donned women in a water perfused suit. In cold air exposures, it would be expected that skin and core temperature would diverge. When using a water perfused suit, stable core and skin temperatures can be maintained such that divergent responses do not occur. Thus, it may be that differences in peripheral thermoreceptor stimulation, a primary driver of shivering thermogenesis, account for the differences in these study findings. Additionally, time of day may also be a contributing factor. The study of Hessemer et al. was performed at night when elevations in core temperature due to menstrual phase are highest, which may have influenced the lack of displacement in the regulated core temperature observed by Blondin et al [Citation110,Citation154].
It should also be noted that thermal perception and discomfort, while drivers of thermal behavior, may also influence autonomic effectors, including shivering. Kaikaew et al. cooled men and women using a water perfused vest and blanket until shivering onset (determined by EMG of the rectus femoris), and found that women required shivering heat production at a higher esophageal temperature [Citation14]. In addition, women perceived identical cooling to be less comfortable and colder than men. In this investigation, the BSA-to-mass ratio did differ between sexes and was a determining factor in shivering onset. However, subgroup analysis of anthropometrically matched men and women suggested that sex was a factor independent of BSA-to-mass ratio. Additionally, thermal comfort (at room temperature) was a determinant of earlier shivering. The authors propose that women may exhaust maximal cutaneous vasoconstrictor capacity sooner and thus require shivering heat production earlier than men [Citation14]. While potential mechanisms for differences in thermal perception were not examined in this investigation, the authors suggested that sex differences in transient receptor potential melastatin 8 (TRPM8) sensitivity may account for differences in cold perception, as observed elsewhere [Citation155].
With regard to shivering activity, there are presently no studies to directly compare shivering activity or pattern between men and women, thus a significant research gap exists. However, shivering activity does not appear to differ across the menstrual cycle. Blondin et al. observed no differences in shivering pattern or shivering intensity between the follicular and luteal phase in mildly cold exposed women [Citation154]. EMG-determined shivering pattern showed no change in burst rate, and shivering intensity remained in a narrow range of ~13–15%MVC between menstrual phases [Citation154].
Shivering Skeletal Muscle Substrate Utilization
Differences in substrate utilization exist between women and men during exercise (as reviewed by Tarnopolsky [Citation156]). During endurance exercise, women oxidize more lipids and less CHO than men, and substrate utilization has been documented to vary between menstrual phases, albeit minimally [Citation157,Citation158]. This metabolic preference toward lipids is facilitated by the actions of estrogens, which increase the expression of fatty acid translocase proteins [Citation156]. This upregulation in turn increases free fatty acid uptake by skeletal muscle and promotes fat oxidation. In addition to a pro-lipid hormonal milieu, women contain more intramyocellular lipid stores than men, which may impart an endurance benefit [Citation159].
The possibility of enhanced lipid metabolism during shivering in women could mean a thermoregulatory advantage for women in the cold [Citation160]. Tikuisis et al. immersed women and men to the neck in 18°C water during glycogen depletion to evaluate if sex differences in substrate utilization should be accounted for in thermoregulatory modeling in relation to extended cold survival. Following immersion, it was concluded that despite differences in rates of cooling between sexes, the metabolic demand was similar and substrate utilization did not differ between the sexes [Citation160]. Comparably, Glickman-Weiss demonstrated that CHO utilization did not differ between women and men when esophageal temperature was reduced to 36.5°C during 20°C water immersion [Citation161].
However, there is also evidence to suggest that sex differences in substrate utilization do exist during shivering. Women exposed to 5°C air for 2 h demonstrated a greater reliance on lipid oxidation than men (64% vs 53%) [Citation162]. Indeed, the respiratory exchange ratio (RER) was lower in women, and men expended more energy on CHO oxidation (47% vs 53%), exhibiting to some extent a glycogen sparing effect in women [Citation162].To provide further evidence, it has been observed from two independent studies conducted by Blondin et al. and Haman et al. that the relative contribution of lipids to total heat production was 25% greater in women than in men at comparable cold intensities (~2.5X RMR) [Citation48,Citation154].
The contradictory outcomes of these studies may be a result a variety of factors, making comparison in outcomes difficult. The above studies employ a range of cooling protocols that result in varying degrees of cold stress. In addition, the influence of body composition is unclear. In the study of Pettit et al., men and women were matched in %body fat and the catecholamine response was similar during cold exposure, suggesting that neither differences in body composition nor sympathetic stimulation contributed to these differences [Citation162]. Rather, Blondin et al. suggested that the usage of different metabolic pathways within the same muscle fiber during shivering muscle recruitment could be a contributing factor in sex differences [Citation154]. Taken together, available evidence suggests that potential sex differences in substrate utilization may be dependent on the severity of cold stress, while the influence of body composition remains uncertain.
With regard to menstrual cycle phase, there appears to be no effect on substrate preference or oxidation rates in shivering skeletal muscle. The aforementioned investigation of Glickman-Weiss et al. reported no influence of the menstrual cycle during water immersion on CHO utilization or RER [Citation161]. Similarly, Tikuisis et al, found no difference in substrate oxidation between the follicular and luteal phases during 90 min of water immersion at 18°C [Citation160]. Moreover, Blondin et al. examined substrate utilization and oxidation rates in mildly cold exposed women (2.5X RMR), and despite increased estradiol and progesterone levels during the luteal phase, there were no differences in substrate selection or oxidation rate [Citation154].
Cold-Induced Thermogenesis
Multiple studies have sought to investigate differences in the metabolic contribution to total heat production between men and women. Pettit et al. exposed men and women to 5°C air for 2 hrs and found no difference in the relative oxygen consumption during cold exposure [Citation162]. Likewise, multiple studies have performed similar investigations using cold water immersion. Tikuisis et al. demonstrated that relative metabolic heat production did not differ between sexes following immersion in 18°C water for up to 90 min [Citation160]. Similarly, it has been demonstrated that the predicted peak shivering response (measured as oxygen consumption) is similar between men and women. Eyolfson et al. immersed men and women in 8°C water for up to 70 min and gradually rewarmed water to 20°C to elicit maximal shivering intensity [Citation37]. While absolute oxygen uptake was higher in men relative to women as expected due to greater muscle mass, relative oxygen consumption was similar between men and women given the same cold stress [Citation37]. Glickman-Weiss et al. described no difference between men and women in relative oxygen consumption during 20°C water immersion [Citation161]. McArdle et al. comparatively immersed men and women in cold water at 20°C, 24°C, and 28°C, and found no differences in oxygen uptake between men and women [Citation163].Taken as a whole, relative cold-induced metabolic responses are comparable between sexes when anthropometric measures are properly controlled [Citation37,Citation160–163].
Furthermore, available evidence suggests that there is no influence of menstrual cycle in cold-induced thermogenesis. In the above studies, Glickman-Weiss et al. and Tikusis et al. reported no influences of menstrual cycle on absolute or relative O2 consumption during cold-induced thermogenesis [Citation160,Citation161], in accordance with other studies explored within this review [Citation110,Citation154].
It is well known that anthropometry and body composition are critical factors in thermoregulatory responses, and that men and women exhibit striking differences in these factors based on sex. In general, women possess more subcutaneous fat, less muscle mass, and smaller BSA than men. While increased subcutaneous fat would be advantageous in insulative protection against heat loss, a lesser amount of muscle mass and larger BSA-to-mass ratio is a disadvantage in a cold environment. In this case, shivering thermogenic potential is smaller and surface area for heat dissipation to the environment larger relative to body size [Citation163]. Therefore, women may experience declines in core temperature sooner than men in environments where ST cannot readily compensate for increased thermal gradients driving heat loss. However, higher adiposity and lower muscle mass may be advantageous for women during exercise in cold water when blood flow to the exercising muscles and periphery is increased.
SEX AND REPRODUCTIVE HORMONE DIFFERENCES IN BAT THERMOGENESIS
Sex Differences in Cold-Induced Brown Adipose Thermogenesis
As discussed previously (see BAT Thermogenesis), retrospective studies originating from the 2009 revitalization of BAT interest reported greater brown adipose mass and 18F-FDG uptake activity in women relative to men [Citation61,Citation80,Citation82,Citation83], while others reported no differences based on sex [Citation81]. In the decade succeeding these initial series of BAT investigations, prospective studies have demonstrated that this observation is debatable. Cypess et al. and Orava et al. revealed no sex difference in BAT mass or activity in young and middle-aged cold exposed men and women [Citation164,Citation165]. Similarly, it has been reported that 18F-FDG detectable BAT mass and activity did not differ between men and women, before or after completion of a 10 day cold acclimation protocol which similarly increased BAT in both sexes [Citation85]. Recently, Fletcher et al. exposed men and women (all in follicular phase) to mild cold [Citation84]. In contrast to the rather concrete notion that women possess more BAT than men, 18F-FDG-determined absolute BAT volume was lower in women, but 18F-FDG uptake was comparable [Citation84].
However, these outcomes in more recent prospective studies that demonstrate a lack of sex differences should not be misconstrued such that reports of higher BAT mass or activity in women do not exist. Indeed, there are investigations that support the finding of enhanced BAT mass and activity in women relative to men [Citation166,Citation167]. Whether a female advantage exists with regard to BAT thermogenesis remains a debated and lingering topic.
Female Sex Hormone Influence in BAT Thermogenesis
The influence of the menstrual cycle on BAT thermogenesis remains largely a mystery. Recently, the first in vivo human study to assess BAT activity across the menstrual cycle was performed by Fuller-Jackson et al., who evaluated BAT heat production using infrared thermal imaging of the supraclavicular region in mildly cold-exposed women (hand immersion at 15°C for 5 min) [Citation168]. Consistent with the known thermoregulatory responses associated with the luteal phase, basal and cold-induced changes in supraclavicular temperature were greater in the luteal phase relative to the follicular phase, and positively correlated with plasma progesterone concentration. Furthermore, during the follicular phase, basal and cold-induced changes in supraclavicular temperature did not differ between men and women [Citation168]. This finding is consistent with another recent controlled cohort study that did not detect differences in BAT activity between women in the follicular phase and men using 18F-FDG PET/CT techniques [Citation84]. Moreover, Fuller-Jackson et al. observed a greater cold-adaptive response (BAT temperature following cessation of cold exposure) in women relative to men, irrespective of menstrual phase, which was positively correlated with 17β-estradiol concentration [Citation168]. It is important to recognize that indirect methods of assessing BAT activity are not fully validated [Citation169]. Thermal imaging techniques and the measurement of supraclavicular skin temperature have increased accessibility in BAT research, as “gold standard” techniques are costly and limited in availability [Citation77]. However, these indirect methods are susceptible to influence by various factors, namely the fact that temperature measurement is limited to the superficial skin as well as the contribution of other thermogenic tissues in proximity to the supraclavicular region [Citation169,Citation170]. Thus, future studies examining the influence of female reproductive hormones on BAT thermogenesis should seek to use more robust methodologies when possible.
The physiological mechanisms in the aforementioned studies are unresolved and more work is needed in humans. From murine models, we have learned that estrogens and progesterone exert BAT enhancing effects, while androgens (e.g. testosterone) promote BAT inhibiting effects [Citation171,Citation172]. Estrogens and progesterone have been shown to positively influence BAT mitochondrial morphology and function [Citation173], sympathetic sensitivity [Citation171,Citation173], lypolytic activity [Citation171], white adipose browning [Citation174], and centrally mediated mechanisms of BAT thermogenesis [Citation175]. These mechanisms can either potentiate (e.g. increase UCP1 content or accrue BAT mass) or enhance BAT activity (e.g. increase SNS outflow and metabolism).
While the roles of estrogens and progesterone are more thoroughly described, follicle stimulating hormone (FSH) may also influence BAT thermogenesis. FSH is a gonadotropic hormone produced by the anterior pituitary gland that stimulates the growth of ovarian follicles [Citation176]. FSH begins to rise at the end of the luteal phase and continues in the early follicular phase, with a rapid rise in the pre-ovulatory phase [Citation177]. In rodents, FSH influences energy metabolism by stimulating adipocyte lipid biosynthesis [Citation178]. Interestingly, there is new evidence to suggest that FSH negatively regulates BAT thermogenesis, such that FSH blockade increases BAT activation and thermogenesis in mice [Citation179]. This finding is supported by data demonstrating that FSH inhibits adipocyte UCP1 expression through reduced cAMP signaling [Citation180]. However, no mechanistic studies examining FSH in humans with regard to BAT or thermoregulation have been performed. Therefore, significant research questions remain to whether there is a potential modulation of thermoregulatory function by FSH across the menstrual cycle.
Collectively, there is strong evidence, albeit predominately in rodent and cell studies as mechanistic studies in humans are lacking, for ovarian hormone influence or regulation in BAT thermogenesis. However, whether direct or indirect, the interactions are complex, and the above examples are certainly not exhaustive. A comprehensive overview of the estrogenic role in BAT physiology is provided in the excellent review by Frank et al. [Citation181].
Brown Adipose Thermogenesis in Women with Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is a reproductive and metabolic syndrome that effects 15% of young women and is a common cause of infertility [Citation182]. The PCOS phenotype is variable, but commonly presents with insulin resistance, obesity, hyperandrogenism, and menstrual cycle irregularities. Exercise regimens are regularly prescribed for PCOS control to improve insulin sensitivity and manage weight. The thermoregulatory challenges in the heat for women with PCOS have been highlighted elsewhere [Citation113], and as a whole, core temperature maintenance is accomplished similarly to women without PCOS [Citation183].
As noted above, estrogen and progesterone promote BAT stimulation, whereas androgens promote BAT inhibition. In recent years, there has been increased interest in evaluating this question in women with PCOS to determine if related obesity and androgenic related factors influence BAT activity. Recent work examining non-cold exposed women with PCOS indicated decreased supraclavicular skin temperature which was inversely correlated with plasma testosterone concentration [Citation184]. This differed from the control group of women without PCOS. Relevant to the current review, mildly cold-exposed women with PCOS demonstrated decreased BAT activity relative to women without PCOS, as revealed using 18F-FDG PET/CT [Citation185]. However, BAT activity in this study was not correlated to plasma testosterone, but rather to BMI and waist circumference. Regardless of the determining factor, it appears that women with PCOS exhibit lower BAT activity, given the limited literature available. Whether this attenuation of BAT activity results in an altered response to cold stress in women with PCOS is unknown.
THERMOEFFECTOR RECRUITMENT AND COORDINATION
Thus far, this review has provided a thorough discussion of distinct cold thermoeffector responses and their modulation by female sex hormones. However, it is important to note that thermoeffectors – cutaneous vasoconstriction, metabolic heat production, and thermal behavior – are recruited in a coordinated manner [Citation9,Citation186–188]. In general, thermoeffectors that consume less energy and resources (i.e., changes in skin blood flow) are initiated prior to those that are more energetically costly (e.g., shivering or sweating) [Citation188,Citation189]. In humans, this notion is supported by observations of cutaneous vasoconstriction prior to the activation of heat-generating responses in the cold [Citation188]. More recent attention has importantly been directed toward investigating the recruitment of thermal behavior and its coordination with autonomic thermoeffectors. Notably, Schlader and colleagues have demonstrated that reductions in skin blood flow during cooling precede thermal behavior (in this case, neck warming), but that individuals engage in thermal behavior prior to any observed increase in metabolism [Citation188], though the order of thermoeffector recruitment may depend on the energy requirements of the behavioral strategies available. Nevertheless, these findings lend support to the idea that thermoeffectors are systematically recruited based on their physiological cost.
To date, there is no available evidence that the order of thermoeffector recruitment is different between men and women or across the menstrual cycle. However, given the influence of sex and reproductive hormones on autonomic thermoeffector responses and thermal perception described above, it is conceivable that the temporal profile of thermoeffector recruitment and the coordination between autonomic and behavioral responses in the cold is modulated by sex. Additional studies that examine the effect of sex on thermoeffector coordination is an important topic of future research.
SUMMARY AND CONCLUSIONS
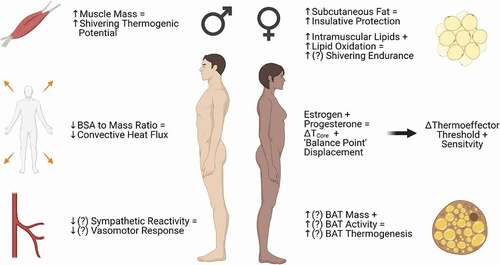
The fluctuations of female reproductive hormones across the menstrual cycle are associated with changes in the control of body temperature overall, and this appears to include shifts in the responses of cutaneous vasoconstriction, shivering, and BAT thermogenesis (). Because of their relatively smaller size and larger surface area-to-mass ratio, women may be seen to have a biophysical disadvantage relative to men in a cold environment. However, the net “advantage” or “disadvantage” appears to be more nuanced than this, including influences of subcutaneous adiposity, differences in temperature preference, substrate utilization during shivering, and other factors. BAT thermogenesis is an intriguing area in this regard, and has received increasing attention in recent years due to its ability to be influenced by ovarian hormones in animal and cellular models. While there may be quantifiable influences of female sex and reproductive hormones on thermoregulatory responses to cold in humans, the specific effects of sex hormones, independent of sex differences in body composition and morphology, remain incompletely understood. Clearly more work is needed to delineate mechanisms of sex differences and reproductive hormone influences on cold thermoregulation, from basic science to practical applications for athletes, the military, and others who spend large amounts of time in cold environments.
Figure 3. Summary of sex differences in the factors influencing heat production and conservation in cold conditions. Outcomes in which data are limited or conflicting are depicted by (?). Compared to men, women tend to possess more subcutaneous fat, which may provide insulative protection against heat loss, but also tend to have a greater surface area for heat dissipation relative to body size. Though these differences in morphology largely explain sex differences in thermoregulatory responses to cold, female sex and reproductive hormones modulate mechanisms of cutaneous vasoconstriction, substrate utilization during shivering, and brown adipose tissue (BAT) activity, which may result in more subtle advantages or disadvantages when exposed to the cold. Figure was created with BioRender.com.
Abbreviations
| Beta-adrenergic receptor | = | (β-AR) |
| Brown adipose tissue | = | (BAT) |
| Body surface area | = | (BSA) |
| Carbohydrate | = | (CHO) |
| Energy expenditure | = | (EE) |
| Follicle stimulating hormone | = | (FSH) |
| Mean body temperature | = | (MBT) |
| Neuropeptide Y | = | (NPY) |
| Nitric oxide | = | (NO) |
| Non-shivering thermogenesis | = | (NST) |
| Norepinephrine | = | (NE) |
| Pre-optic anterior hypothalamus | = | (PO/AH) |
| Polycystic ovarian syndrome | = | (PCOS) |
| Respiratory exchange ratio | = | (RER) |
| Rho-kinase | = | (ROCK) |
| Reactive oxygen species | = | (ROS) |
| Shivering thermogenesis | = | (ST) |
| Sympathetic nervous system | = | (SNS) |
| Uncoupling protein | = | (UCP) |
Disclaimers
The views, opinions, and/or findings contained in this article are those of the authors and should not be construed as an official United States Department of the Army position, or decision, unless so designated by other official documentation. This article is approved for public release, and distribution is unlimited.
Citations of commercial organizations and trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
This research was supported in part by an appointment to the Department of Defense (DOD) Research Participation Program administered by the Oak Ridge Institute for Science and Education (ORISE) through an interagency agreement between the U.S. Department of Energy (DOE) and the DOD. ORISE is managed by ORAU under DOE contract number DE-SC0014664. All opinions expressed in this paper are the author’s and do not necessarily reflect the policies and views of the U.S. Army, DOD, DOE, or ORAU/ORISE.
Author contributions
All authors (A.M.G., N.C., and B.K.A) contributed to drafting and revising the manuscript, and all authors approved the final version.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Halbrecht I. Ovarian function and body temperature. Lancet. 1945;246(6378):668.
- Marshall J. Thermal Changes in the Normal Menstrual Cycle. Br Med J. 1963;1(5323):102–104.
- Brooks EM, Morgan AL, Pierzga JM, et al. Chronic hormone replacement therapy alters thermoregulatory and vasomotor function in postmenopausal women. J Appl Physiol (1985). 1997;83(2):477–484.
- Krajewski-Hall SJ, Blackmore EM, McMinn JR, et al. Estradiol alters body temperature regulation in the female mouse. Temperature. 2017; 5(1):56–69. doi:https://doi.org/10.1080/23328940.2017.1384090
- Silva NL, Boulant JA. Effects of testosterone, estradiol, and temperature on neurons in preoptic tissue slices. Am J Physiol. 1986;250:R625–632.
- Stephenson LA, Kolka MA. Esophageal temperature threshold for sweating decreases before ovulation in premenopausal women. J Appl Physiol (1985). 1999;86(1):22–28.
- Gagge AP, Stolwijk JA, Hardy JD. Comfort and thermal sensations and associated physiological responses at various ambient temperatures. Environ Res. 1967;1(1):1–20.
- Schlader ZJ, Simmons SE, Stannard SR, et al. The independent roles of temperature and thermal perception in the control of human thermoregulatory behavior. Physiol Behav. 2011;103(2):217–224.
- Schlader ZJ, Vargas NT. Regulation of Body Temperature by Autonomic and Behavioral Thermoeffectors. Exerc Sport Sci Rev. 2019;47(2):116–126.
- Gerrett N, Ouzzahra Y, Coleby S, et al. Thermal sensitivity to warmth during rest and exercise: a sex comparison. Eur J Appl Physiol. 2014;114(7):1451–1462.
- Golja P, Tipton MJ, Mekjavic IB. Cutaneous thermal thresholds—the reproducibility of their measurements and the effect of gender. J Therm Biol. 2003;28(4):341–346.
- Schoech L, Allie K, Salvador P, et al. Sex Differences in Thermal Comfort, Perception, Feeling, Stress and Focus During Exercise Hyperthermia. Percept Mot Skills. 2021;128(3):969–987.
- Beshir MY, Ramsey JD. Comparison between male and female subjective estimates of thermal effects and sensations. Appl Ergon. 1981;12(1):29–33.
- Kaikaew K, van den Beukel JC, Neggers S, et al. Sex difference in cold perception and shivering onset upon gradual cold exposure. J Therm Biol. 2018;77:137–144.
- Kenshalo DR. Changes in the cool threshold associated with phases of the menstrual cycle. J Appl Physiol. 1966;21(3):1031–1039.
- Kim HE, Tokura H. Effects of the menstrual cycle on dressing behavior in the cold. Physiol Behav. 1995;58:699–703.
- Charkoudian N. Skin blood flow in adult human thermoregulation: how it works, when it does not, and why. Mayo Clin Proc. 2003;78(5):603–612.
- Johnson JM, Kellogg DL Jr. Skin vasoconstriction as a heat conservation thermoeffector. Handb Clin Neurol. 2018;156:175–192.
- Blondin DP, Haman F. Shivering and nonshivering thermogenesis in skeletal muscles. Handb Clin Neurol. 2018;156(153–173).
- Haman F, Blondin DP. Shivering thermogenesis in humans: origin, contribution and metabolic requirement. Temperature. 2017;4(3):217–226. doi:https://doi.org/10.1080/23328940.2017.1328999
- Alvarez GE, Zhao K, Kosiba WA, et al. Relative roles of local and reflex components in cutaneous vasoconstriction during skin cooling in humans. J Appl Physiol (1985). 2006;100:2083–2088.
- Johnson JM, Minson CT, Kellogg DL Jr. Cutaneous vasodilator and vasoconstrictor mechanisms in temperature regulation. Compr Physiol. 2014;4:33–89.
- Stephens DP, Aoki K, Kosiba WA, et al. Nonnoradrenergic mechanism of reflex cutaneous vasoconstriction in men. Am J Physiol Heart Circ Physiol. 2001;280(4):H1496–H1504.
- Thompson CS, Kenney WL. Altered neurotransmitter control of reflex vasoconstriction in aged human skin. J Physiol. 2004;558(2):697–704.
- Lang JA, Krajek AC, Smaller KA. Evidence for a functional vasoconstrictor role for ATP in the human cutaneous microvasculature. Exp Physiol. 2017;102:684–693.
- Stephens DP, Saad AR, Bennett LA, et al. Neuropeptide Y antagonism reduces reflex cutaneous vasoconstriction in humans. Am J Physiol Heart Circ Physiol. 2004;287(3):H1404–H1409.
- Lang JA, Jennings JD, Holowatz LA, et al. Reflex vasoconstriction in aged human skin increasingly relies on Rho kinase-dependent mechanisms during whole body cooling. Am J Physiol Heart Circ Physiol. 2009;297(6):H1792–H1797.
- Bailey SR, Eid AH, Mitra S, et al. Rho kinase mediates cold-induced constriction of cutaneous arteries: role of alpha2C-adrenoceptor translocation. Circ Res. 2004;94(10):1367–1374.
- Hodges GJ, Zhao K, Kosiba WA, et al. The involvement of nitric oxide in the cutaneous vasoconstrictor response to local cooling in humans. J Physiol. 2006;574(3):849–857.
- Johnson JM, Yen TC, Zhao K, et al. Sympathetic, sensory, and nonneuronal contributions to the cutaneous vasoconstrictor response to local cooling. Am J Physiol Heart Circ Physiol. 2005;288(4):H1573–1579.
- Chotani MA, Flavahan S, Mitra S, et al. Silent α2 adrenergic receptors enable cold-induced vasoconstriction in cutaneous arteries. Am J Physiol Heart Circ Physiol. 2000;278(4):H1075–H1083.
- Bailey SR, Mitra S, Flavahan S, et al. Reactive oxygen species from smooth muscle mitochondria initiate cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol. 2005;289(1):H243–H250.
- Thompson-Torgerson CS, Holowatz LA, Flavahan NA, et al. Cold-induced cutaneous vasoconstriction is mediated by Rho kinase in vivo in human skin. Am J Physiol Heart Circ Physiol. 2007;292(4):H1700–H1705.
- Yamazaki F. Local ascorbate administration inhibits the adrenergic vasoconstrictor response to local cooling in the human skin. J Appl Physiol (1985). 2010;108(2):328–333.
- Torii M. Maximal sweating rate in humans. J Hum Ergol (Tokyo). 1995;24(137–152).
- Wenger CB. Heat of evaporation of sweat: thermodynamic considerations. J Appl Physiol. 1972;32(4):456–459.
- Eyolfson DA, Tikuisis P, Xu X, et al. Measurement and prediction of peak shivering intensity in humans. Eur J Appl Physiol. 2001;84(1–2):100–106.
- Bergh U, Ekblom B. Physical performance and peak aerobic power at different body temperatures. J Appl Physiol Respir Environ Exerc Physiol. 1979;46(885–889).
- Nadel ER, Wenger CB, Roberts MF, et al. Physiological defenses against hyperthermia of exercise. Ann N Y Acad Sci. 1977;301(1 The Marathon):98–109.
- Bell DG, Tikuisis P, Jacobs I. Relative intensity of muscular contraction during shivering. J Appl Physiol (1985). 1992;72(6):2336–2342.
- Betz MJ, Enerback S. Targeting thermogenesis in brown fat and muscle to treat obesity and metabolic disease. Nat Rev Endocrinol. 2018;14(2):77–87.
- van Marken Lichtenbelt WD, Schrauwen P. Implications of nonshivering thermogenesis for energy balance regulation in humans. Am J Physiol Regul Integr Comp Physiol. 2011;301(2):R285–R296.
- Morrison SF. Central neural control of thermoregulation and brown adipose tissue. Auton Neurosci. 2016;196(14):14–24.
- Schafer SS, Schafer S. The behavior of the proprioceptors of the muscle and the innervation of the fusimotor system during cold shivering. Exp Brain Res. 1973;17(4). DOI:https://doi.org/10.1007/BF00234100
- Castellani JW, Young AJ. Human physiological responses to cold exposure: acute responses and acclimatization to prolonged exposure. Auton Neurosci. 2016;196(63):63–74.
- Gonzalez RR, Blanchard LA. Thermoregulatory responses to cold transients: effects of menstrual cycle in resting women. J Appl Physiol (1985). 1998;85(2):543–553.
- Vallerand AL, Jacobs I. Rates of energy substrates utilization during human cold exposure. Eur J Appl Physiol Occup Physiol. 1989;58(8):873–878.
- Haman F, Peronnet F, Kenny GP, et al. Partitioning oxidative fuels during cold exposure in humans: muscle glycogen becomes dominant as shivering intensifies. J Physiol. 2005;566(1):247–256.
- Haman F, Scott CG, Kenny GP. Fueling shivering thermogenesis during passive hypothermic recovery. J Appl Physiol (1985). 2007;103(4):1346–1351.
- Haman F, Legault SR, Rakobowchuk M, et al. Effects of carbohydrate availability on sustained shivering II. Relating muscle recruitment to fuel selection. J Appl Physiol (1985). 2004;96(1):41–49.
- Haman F, Peronnet F, Kenny GP, et al. Effects of carbohydrate availability on sustained shivering I. Oxidation of plasma glucose, muscle glycogen, and proteins. J Appl Physiol (1985). 2004;96(1):32–40.
- Haman F. Shivering in the cold: from mechanisms of fuel selection to survival. J Appl Physiol (1985). 2006;100(5):1702–1708.
- Blondin DP, Frisch F, Phoenix S, et al. Inhibition of Intracellular Triglyceride Lipolysis Suppresses Cold-Induced Brown Adipose Tissue Metabolism and Increases Shivering in Humans. Cell Metab. 2017;25(2):438–447.
- Gordon K, Blondin DP, Friesen BJ, et al. Seven days of cold acclimation substantially reduces shivering intensity and increases nonshivering thermogenesis in adult humans. J Appl Physiol (1985). 2019;126(6):1598–1606.
- Gessner K. Conradi Gesneri medici Tigurine Historae Animalium: lib. I De Quadrupedibus viviparis. 1551.
- Smith RE, Horwitz BA. Brown fat and thermogenesis. Physiol Rev. 1969;49(330–425).
- Aherne W, Hull D. Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol. 1966;91(1):223–234.
- Nedergaard J, Cannon B. The changed metabolic world with human brown adipose tissue: therapeutic visions. Cell Metab. 2010;11(4):268–272.
- Davis TR. Chamber cold acclimatization in man. J Appl Physiol. 1961;16(6):1011–1015.
- Huttunen P, Hirvonen J, Kinnula V. The occurrence of brown adipose tissue in outdoor workers. Eur J Appl Physiol Occup Physiol. 1981;46(4):339–345.
- Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–1517.
- Saito M, Okamatsu-Ogura Y, Matsushita M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes. 2009;58(7):1526–1531.
- van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360(15):1500–1508.
- Virtanen KA, Lidell ME, Orava J, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360(15):1518–1525.
- Ouellet V, Labbe SM, Blondin DP, et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J Clin Invest. 2012;122(2):545–552.
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84(277–359).
- Matthias A, Ohlson KB, Fredriksson JM, et al. Thermogenic responses in brown fat cells are fully UCP1-dependent. UCP2 or UCP3 do not substitute for UCP1 in adrenergically or fatty scid-induced thermogenesis. J Biol Chem. 2000;275(33):25073–25081.
- Lee P, Swarbrick MM, Zhao JT, et al. Inducible brown adipogenesis of supraclavicular fat in adult humans. Endocrinology. 2011;152(10):3597–3602.
- Lever JD, Nnodim JO, Symons D. Arteriovenous anastomoses in interscapular brown adipose tissue in the rat. J Anat. 1985;143(207–210).
- Thoonen R, Hindle AG, Scherrer-Crosbie M. Brown adipose tissue: the heat is on the heart. Am J Physiol Heart Circ Physiol. 2016;310(11):H1592–H1605.
- Zingaretti MC, Crosta F, Vitali A, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23(9):3113–3120.
- Lowell BB, Flier JS. Brown adipose tissue, beta 3-adrenergic receptors, and obesity. Annu Rev Med. 1997;48(1):307–316.
- Zhao J, Cannon B, Nedergaard J. Thermogenesis is beta3- but not beta1-adrenergically mediated in rat brown fat cells, even after cold acclimation. Am J Physiol. 1998;275:R2002–2011.
- Blondin DP, Nielsen S, Kuipers EN, et al. Human Brown Adipocyte Thermogenesis Is Driven by beta2-AR Stimulation. Cell Metab. 2020;32(2):e287.
- Riis-Vestergaard MJ, Richelsen B, Bruun JM, et al. Beta-1 and Not Beta-3 Adrenergic Receptors May Be the Primary Regulator of Human Brown Adipocyte Metabolism. J Clin Endocrinol Metab. 2020;105(4):e994–e1005.
- Kuusela P, Nedergaard J, Cannon B. Beta-adrenergic stimulation of fatty acid release from brown fat cells differentiated in monolayer culture. Life Sci. 1986;38(2):589–599.
- Richard MA, Pallubinsky H, Blondin DP. Functional characterization of human brown adipose tissue metabolism. Biochem J. 2020;477(7):1261–1286.
- Carpentier AC, Blondin DP, Virtanen KA, et al. Brown Adipose Tissue Energy Metabolism in Humans. Front Endocrinol (Lausanne). 2018;9(447). DOI:https://doi.org/10.3389/fendo.2018.00447
- Morrison SF, Madden CJ, Tupone D. Central control of brown adipose tissue thermogenesis. Front Endocrinol (Lausanne). 2012;3. DOI:https://doi.org/10.3389/fendo.2012.00005
- Ouellet V, Routhier-Labadie A, Bellemare W, et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J Clin Endocrinol Metab. 2011;96(1):192–199.
- Yoneshiro T, Aita S, Matsushita M, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring). 2011;19(9):1755–1760.
- Au-Yong IT, Thorn N, Ganatra R, et al. Brown adipose tissue and seasonal variation in humans. Diabetes. 2009;58(11):2583–2587.
- Pfannenberg C, Werner MK, Ripkens S, et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes. 2010;59(7):1789–1793.
- Fletcher LA, Kim K, Leitner BP, et al. Sexual Dimorphisms in Adult Human Brown Adipose Tissue. Obesity (Silver Spring). 2020;28(2):241–246.
- van der Lans AA, Hoeks J, Brans B, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J Clin Invest. 2013;123(8):3395–3403.
- van der Lans AA, Wierts R, Vosselman MJ, et al. Cold-activated brown adipose tissue in human adults: methodological issues. Am J Physiol Regul Integr Comp Physiol. 2014;307(2):R103–R113.
- Kingma B, Frijns A, van Marken Lichtenbelt W. The thermoneutral zone: implications for metabolic studies. Front Biosci (Elite Ed). 2012;4(5):1975–1985.
- Blondin DP, Daoud A, Taylor T, et al. Four-week cold acclimation in adult humans shifts uncoupling thermogenesis from skeletal muscles to brown adipose tissue. J Physiol. 2017;595(6):2099–2113.
- Blondin DP, Labbe SM, Tingelstad HC, et al. Increased brown adipose tissue oxidative capacity in cold-acclimated humans. J Clin Endocrinol Metab. 2014;99(3):E438–E446.
- Blondin DP, Tingelstad HC, Noll C, et al. Dietary fatty acid metabolism of brown adipose tissue in cold-acclimated men. Nat Commun. 2017;8(1). DOI:https://doi.org/10.1038/ncomms14146
- Yoneshiro T, Aita S, Matsushita M, et al. Recruited brown adipose tissue as an antiobesity agent in humans. J Clin Invest. 2013;123(8):3404–3408.
- Rowland LA, Bal NC, Periasamy M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol Rev Camb Philos Soc. 2015;90(4):1279–1297.
- Bal NC, Periasamy M. Uncoupling of sarcoendoplasmic reticulum calcium ATPase pump activity by sarcolipin as the basis for muscle non-shivering thermogenesis. Philos Trans R Soc Lond B Biol Sci. 2020;375(20190135).
- Periasamy M, Maurya SK, Sahoo SK, et al. Role of SERCA Pump in Muscle Thermogenesis and Metabolism. Compr Physiol. 2017;7(879–890).
- Mailloux RJ, Seifert EL, Bouillaud F, et al. Glutathionylation acts as a control switch for uncoupling proteins UCP2 and UCP3. J Biol Chem. 2011;286(24):21865–21875.
- Harper ME, Dent R, Monemdjou S, et al. Decreased mitochondrial proton leak and reduced expression of uncoupling protein 3 in skeletal muscle of obese diet-resistant women. Diabetes. 2002;51(8):2459–2466.
- Wijers SL, Schrauwen P, Saris WH, et al. Human skeletal muscle mitochondrial uncoupling is associated with cold induced adaptive thermogenesis. PLoS One. 2008;3(3):e1777.
- Wijers SL, Schrauwen P, van Baak MA, et al. Beta-adrenergic receptor blockade does not inhibit cold-induced thermogenesis in humans: possible involvement of brown adipose tissue. J Clin Endocrinol Metab. 2011;96(4):E598–E605.
- UD M, Raiko J, Saari T, et al. Human brown adipose tissue [(15)O]O2 PET imaging in the presence and absence of cold stimulus. Eur J Nucl Med Mol Imaging. 2016;43(10):1878–1886.
- Kim J, Wang Z, Heymsfield SB, et al. Total-body skeletal muscle mass: estimation by a new dual-energy X-ray absorptiometry method. Am J Clin Nutr. 2002;76(2):378–383.
- Cole LA, Ladner DG, Byrn FW. The normal variabilities of the menstrual cycle. Fertil Steril. 2009;91(2):522–527.
- Faust L, Bradley D, Landau E, et al. Findings from a mobile application-based cohort are consistent with established knowledge of the menstrual cycle, fertile window, and conception. Fertil Steril. 2019;112(450–457):e453.
- Sims ST, Heather AK. Myths and Methodologies: reducing scientific design ambiguity in studies comparing sexes and/or menstrual cycle phases. Exp Physiol. 2018;103(10):1309–1317.
- Baker FC, Siboza F, Fuller A. Temperature regulation in women: effects of the menstrual cycle. Temperature. 2020; 7(3):226–262. doi:https://doi.org/10.1080/23328940.2020.1735927
- Stachenfeld NS, Taylor HS. Challenges and methodology for testing young healthy women in physiological studies. Am J Physiol Endocrinol Metab. 2014;306(8):E849–E853.
- Stachenfeld NS, Taylor HS. Sex hormone effects on body fluid and sodium regulation in women with and without exercise-associated hyponatremia. J Appl Physiol (1985). 2009;107(3):864–872.
- Gibbs JC, Williams NI, Mallinson RJ, et al. and De Souza MJ. Effect of high dietary restraint on energy availability and menstrual status. Med Sci Sports Exerc. 2013;45(9):1790–1797.
- Williams NI, Leidy HJ, Hill BR, et al. Magnitude of daily energy deficit predicts frequency but not severity of menstrual disturbances associated with exercise and caloric restriction. Am J Physiol Endocrinol Metab. 2015;308(1):E29–E39.
- Barnes JN, Hart EC, Curry TB, et al. Aging enhances autonomic support of blood pressure in women. Hypertension. 2014;63(2):303–308.
- Hessemer V, Bruck K. Influence of menstrual cycle on shivering, skin blood flow, and sweating responses measured at night. J Appl Physiol (1985). 1985;59(6):1902–1910.
- Kolka MA, Stephenson LA. Control of sweating during the human menstrual cycle. Eur J Appl Physiol Occup Physiol. 1989;58(8):890–895.
- Stephenson LA, Kolka MA. Menstrual cycle phase and time of day alter reference signal controlling arm blood flow and sweating. Am J Physiol. 1985;249(R186–191).
- Charkoudian N, Stachenfeld N. Sex hormone effects on autonomic mechanisms of thermoregulation in humans. Auton Neurosci. 2016;196(75):75–80.
- Charkoudian N, Johnson JM. Altered reflex control of cutaneous circulation by female sex steroids is independent of prostaglandins. Am J Physiol. 1999;276(H1634–1640).
- Tsai CL, Matsumura K, Nakayama T. Effects of progesterone on thermosensitive neurons in preoptic slice preparations. Neurosci Lett. 1988;86(1):56–60.
- Johnson JM, Proppe DW. Cardiovascular adjustments to heat stress. In: Handbook of Physiology: environmental Physiology. New York: Oxford University Press; 1996. p. 215–244.
- Frascarolo P, Schutz Y, Jequier E. Decreased thermal conductance during the luteal phase of the menstrual cycle in women. J Appl Physiol (1985). 1990;69(6):2029–2033.
- Savage MV, Brengelmann GL. Control of skin blood flow in the neutral zone of human body temperature regulation. J Appl Physiol (1985). 1996;80(4):1249–1257.
- Charkoudian N, Johnson JM. Female reproductive hormones and thermoregulatory control of skin blood flow. Exerc Sport Sci Rev. 2000;28(108–112).
- Davidson L, Rouse IL, Vandongen R, et al. Plasma noradrenaline and its relationship to plasma oestradiol in normal women during the menstrual cycle. Clin Exp Pharmacol Physiol. 1985;12(5):489–493.
- Minson CT, Halliwill JR, Young TM, et al. Influence of the menstrual cycle on sympathetic activity, baroreflex sensitivity, and vascular transduction in young women. Circulation. 2000;101(8):862–868.
- Ferrer M, Osol G. Estrogen Replacement Modulates Resistance Artery Smooth Muscle and Endothelial α2 Adrenoceptor Reactivity. Endothelium. 1998;6(2):133–141.
- Meyer MC, Cummings K, Osol G. Estrogen replacement attenuates resistance artery adrenergic sensitivity via endothelial vasodilators. Am J Physiol. 1997;272(H2264–2270).
- O’Conner JL, Wade MF, Brann DW, et al. Evidence that progesterone modulates anterior pituitary neuropeptide Y levels during the progesterone-induced gonadotropin surge in the estrogen-primed intact immature female rat. J Steroid Biochem Mol Biol. 1995;52(497–504).
- Parker SL, Carroll BL, Kalra SP, et al. Neuropeptide Y Y2 receptors in hypothalamic neuroendocrine areas are up-regulated by estradiol and decreased by progesterone cotreatment in the ovariectomized rat. Endocrinology. 1996;137(7):2896–2900.
- Eid AH, Maiti K, Mitra S, et al. Estrogen increases smooth muscle expression of α2C adrenoceptors and cold-induced constriction of cutaneous arteries. Am J Physiol Heart Circ Physiol. 2007;293(3):H1955–1961.
- Freedman RR, Girgis R. Effects of menstrual cycle and race on peripheral vascular alpha-adrenergic responsiveness. Hypertension. 2000;35(3):795–799.
- Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60(2):210–241.
- Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20(9):1996–2009.
- Sudhir K, Jennings GL, Funder JW, et al. Estrogen enhances basal nitric oxide release in the forearm vasculature in perimenopausal women. Hypertension. 1996;28(3):330–334.
- Wenner MM, Taylor HS, Stachenfeld NS. Progesterone enhances adrenergic control of skin blood flow in women with high but not low orthostatic tolerance. J Physiol. 2011;589(4):975–986.
- Charkoudian N, Johnson JM. Reflex control of cutaneous vasoconstrictor system is reset by exogenous female reproductive hormones. J Appl Physiol (1985). 1999;87(1):381–385.
- Charkoudian N, Stephens DP, Pirkle KC, et al. Influence of female reproductive hormones on local thermal control of skin blood flow. J Appl Physiol (1985). 1999;87(5):1719–1723.
- Stephens DP, Bennett LA, Aoki K, et al. Sympathetic nonnoradrenergic cutaneous vasoconstriction in women is associated with reproductive hormone status. Am J Physiol Heart Circ Physiol. 2002;282(1):H264–H272.
- Xu M, Urban JH, Hill JW, et al. Regulation of hypothalamic neuropeptide Y Y1 receptor gene expression during the estrous cycle: role of progesterone receptors. Endocrinology. 2000;141(9):3319–3327.
- Darney PD. The androgenicity of progestins. Am J Med. 1995;98(1):104S–110S.
- Sitruk-Ware R. New progestagens for contraceptive use. Hum Reprod Update. 2006;12(2):169–178.
- Coelingh Bennink HJT, Zimmerman Y, Laan E, et al. and van Lunsen RHW. Maintaining physiological testosterone levels by adding dehydroepiandrosterone to combined oral contraceptives: i. Endocrine effects. Contraception. 2017;96(5):322–329.
- Giribela CR, Consolim-Colombo FM, Nisenbaum MG, et al. Effects of a combined oral contraceptive containing 20 mcg of ethinylestradiol and 3 mg of drospirenone on the blood pressure, renin-angiotensin-aldosterone system, insulin resistance, and androgenic profile of healthy young women. Gynecol Endocrinol. 2015;31(11):912–915.
- Zimmerman Y, Eijkemans MJ, Coelingh Bennink HJ, et al. The effect of combined oral contraception on testosterone levels in healthy women: a systematic review and meta-analysis. Hum Reprod Update. 2014;20(1):76–105.
- Daanen HA. Finger cold-induced vasodilation: a review. Eur J Appl Physiol. 2003;89(5):411–426.
- Hurley HJ Jr., Mescon H. Cholinergic innervation of the digital arteriovenous anastomoses of human skin; a histochemical localization of cholinesterase. J Appl Physiol. 1956;9(1):82–84.
- Hurley HJ Jr., Mescon H, Moretti G. The anatomy and histochemistry of the arteriovenous anastomosis in human digital skin. J Invest Dermatol. 1956;27(3):133–145.
- Cooke JP, Marshall JM. Mechanisms of Raynaud’s disease. Vasc Med. 2005;10(4):293–307.
- Gifford RW Jr., Hines EA Jr. Raynaud’s disease among women and girls. Circulation. 1957;16(6):1012–1021.
- Block JA, Sequeira W. Raynaud’s phenomenon. Lancet. 2001;357(9273):2042–2048.
- Fraenkel L, Zhang Y, Chaisson CE, et al. The association of estrogen replacement therapy and the Raynaud phenomenon in postmenopausal women. Ann Intern Med. 1998;129(3):208–211.
- Cankar K, Finderle Ž. Gender differences in cutaneous vascular and autonomic nervous response to local cooling. Clin Auton Res. 2003;13(3):214–220.
- Cankar K, Finderle Z, Strucl M. Gender differences in cutaneous laser doppler flow response to local direct and contralateral cooling. J Vasc Res. 2000;37(183–188).
- Cankar K, Finderle Z, Strucl M. The role of alpha1- and alpha2-adrenoceptors in gender differences in cutaneous LD flux response to local cooling. Microvasc Res. 2004;68(2):126–131.
- Bartelink ML, De Wit A, Wollersheim H, et al. Skin vascular reactivity in healthy subjects: influence of hormonal status. J Appl Physiol (1985). 1993;74(2):727–732.
- Cankar K, Music M, Finderle Z. Cutaneous microvascular response during local cold exposure - the effect of female sex hormones and cold perception. Microvasc Res. 2016;108(34):34–40.
- Tyler CJ, Reeve T, Cheung SS. Cold-induced vasodilation during single digit immersion in 0 degrees C and 8 degrees C water in men and women. PLoS One. 2015;10(4):e0122592.
- Blondin DP, Maneshi A, Imbeault MA, et al. Effects of the menstrual cycle on muscle recruitment and oxidative fuel selection during cold exposure. J Appl Physiol (1985). 2011;111(4):1014–1020.
- Caudle RM, Caudle SL, Jenkins AC, et al. Sex differences in mouse Transient Receptor Potential Cation Channel, Subfamily M, Member 8 expressing trigeminal ganglion neurons. PLoS One. 2017;12(5):e0176753.
- Tarnopolsky MA. Sex differences in exercise metabolism and the role of 17-beta estradiol. Med Sci Sports Exerc. 2008;40(4):648–654.
- Casazza GA, Jacobs KA, Suh SH, et al. Menstrual cycle phase and oral contraceptive effects on triglyceride mobilization during exercise. J Appl Physiol (1985). 2004;97(1):302–309.
- Zderic TW, Coggan AR, Ruby BC. Glucose kinetics and substrate oxidation during exercise in the follicular and luteal phases. J Appl Physiol (1985). 2001;90(2):447–453.
- Roepstorff C, Steffensen CH, Madsen M, et al. Gender differences in substrate utilization during submaximal exercise in endurance-trained subjects. Am J Physiol Endocrinol Metab. 2002;282(2):E435–E447.
- Tikuisis P, Jacobs I, Moroz D, et al. Comparison of thermoregulatory responses between men and women immersed in cold water. J Appl Physiol (1985). 2000;89(4):1403–1411.
- Glickman-Weiss E, Caine N, Cheatham CC, et al. The effects of gender and menstrual phase on carbohydrate utilization during acute cold exposure. Wilderness Environ Med. 2000;11(1):5–11.
- Pettit SE, Marchand I, Graham T. Gender differences in cardiovascular and catecholamine responses to cold-air exposure at rest. Can J Appl Physiol. 1999;24(2):131–147.
- McArdle WD, Magel JR, Gergley TJ, et al. Thermal adjustment to cold-water exposure in resting men and women. J Appl Physiol Respir Environ Exerc Physiol. 1984;56(1565–1571).
- Cypess AM, Chen YC, Sze C, et al. Cold but not sympathomimetics activates human brown adipose tissue in vivo. Proc Natl Acad Sci U S A. 2012;109(25):10001–10005.
- Orava J, Nuutila P, Lidell ME, et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 2011;14(2):272–279.
- Mihalopoulos NL, Yap JT, Beardmore B, et al. Cold-Activated Brown Adipose Tissue is Associated with Less Cardiometabolic Dysfunction in Young Adults with Obesity. Obesity (Silver Spring). 2020;28(5):916–923.
- Muzik O, Mangner TJ, Granneman JG. Assessment of oxidative metabolism in brown fat using PET imaging. Front Endocrinol (Lausanne). 2012;3(15). DOI:https://doi.org/10.3389/fendo.2012.00015
- Fuller-Jackson JP, Dordevic AL, Clarke IJ, et al. Effect of sex and sex steroids on brown adipose tissue heat production in humans. Eur J Endocrinol. 2020;183(3):343–355.
- Jimenez-Pavon D, Corral-Perez J, Sanchez-Infantes D, et al. Infrared Thermography for Estimating Supraclavicular Skin Temperature and BAT Activity in Humans: a Systematic Review. Obesity (Silver Spring). 2019;27(12):1932–1949.
- Law J, Chalmers J, Morris DE, et al. The use of infrared thermography in the measurement and characterization of brown adipose tissue activation. Temperature (Austin). 2018; 5(2):147–161. doi:https://doi.org/10.1080/23328940.2017.1397085
- Monjo M, Rodriguez AM, Palou A, et al. Direct effects of testosterone, 17 beta-estradiol, and progesterone on adrenergic regulation in cultured brown adipocytes: potential mechanism for gender-dependent thermogenesis. Endocrinology. 2003;144(11):4923–4930.
- Rodriguez AM, Monjo M, Roca P, et al. Opposite actions of testosterone and progesterone on UCP1 mRNA expression in cultured brown adipocytes. Cell Mol Life Sci. 2002;59(10):1714–1723.
- Rodriguez-Cuenca S, Pujol E, Justo R, et al. Sex-dependent thermogenesis, differences in mitochondrial morphology and function, and adrenergic response in brown adipose tissue. J Biol Chem. 2002;277(45):42958–42963.
- van den Beukel JC, Grefhorst A, Hoogduijn MJ, et al. Women have more potential to induce browning of perirenal adipose tissue than men. Obesity (Silver Spring). 2015;23(8):1671–1679.
- Martinez de Morentin PB, Gonzalez-Garcia I, Martins L, et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20(1):41–53. .
- Simoni M, Gromoll J, Nieschlag E. The follicle-stimulating hormone receptor: biochemistry, molecular biology, physiology, and pathophysiology. Endocr Rev. 1997;18:739–773.
- Reed BG, Carr BR. The Normal Menstrual Cycle and the Control of Ovulation. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth (MA); 2000.
- Sponton CH, Kajimura S. Burning Fat and Building Bone by FSH Blockade. Cell Metab. 2017;26(2):285–287.
- Liu P, Ji Y, Yuen T, et al. FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107–112.
- Liu XM, Chan HC, Ding GL, et al. FSH regulates fat accumulation and redistribution in aging through the Gαi/Ca2+/CREB pathway. Aging Cell. 2015;14(3):409–420.
- Frank AP, Palmer BF, Clegg DJ. Do estrogens enhance activation of brown and beiging of adipose tissues? Physiol Behav. 2018;187:24–31.
- Vrbikova J, Hainer V. Obesity and polycystic ovary syndrome. Obes Facts. 2009;2(1):26–35.
- Stachenfeld NS, Yeckel CW, Taylor HS. Greater exercise sweating in obese women with polycystic ovary syndrome compared with obese controls. Med Sci Sports Exerc. 2010;42(9):1660–1668.
- Shorakae S, Jona E, De Courten B, et al. Brown adipose tissue thermogenesis in polycystic ovary syndrome. Clin Endocrinol (Oxf). 2019;90(425–432).
- Oliveira FR, Mamede M, Bizzi MF, et al. Brown adipose tissue activity is reduced in women with polycystic ovary syndrome. Eur J Endocrinol. 2019;181(473–480).
- Romanovsky AA. The thermoregulation system and how it works. In: Romanovsky AA, editor. Handbook of Clinical Neurology. Elsevier; 2018; 156:3–43.
- Schlader ZJ, Coleman GL, Sackett JR, et al. Activation of autonomic thermoeffectors preceding the decision to behaviourally thermoregulate in resting humans. Exp Physiol. 2016;101(9):1218–1229.
- Schlader ZJ, Sackett JR, Sarker S, et al. Orderly recruitment of thermoeffectors in resting humans. Am J Physiol Regul Integr Comp Physiol. 2018;314(2):R171–R180.
- Vargas NT, Schlader ZJ. Physiological benefits likely underlie the systematic recruitment of thermoeffectors. Temperature. 2018; 5(3):199–201. doi:https://doi.org/10.1080/23328940.2017.1415094
