Abstract
Stress fractures at the tibial diaphysis are prone to delayed union. We hypothesized that the interfragmentary strains in the gap tissue are of magnitudes that prohibit bone formation and healing. If so, a rational treatment would be to widen the fracture gap in order to decrease the strains. This could be achieved by replacing part of the fracture crack with a wide drilled hole. This study analyzes the biomechanical effects of this potential treatment through computational modeling. Poroelastic finite element models of an intact tibia, a tibia with a stress fracture, and a tibia with a drilled stress fracture were developed from clinical CT images. Loads corresponding to gait and stair climbing were simulated. Stresses, strains, and fluid velocities were evaluated for the bone in the tibia, and for the hypothetical soft tissue that fills the stress fracture. A minor decrease in the overall tibial stiffness (<9.9%) was noted as a result of drilling. The models predicted large interfragmentary strains in the soft gap tissue (max 35%) before drilling, which decreased substantially (<1%) after drilling. Thus, when comparing to current mechanobiological tissue differentiation theories, the authors believe that fracture gap widening by drilling a hole might facilitate healing of stress fractures.
Introduction
A stress fracture is a partial or complete fracture of a bone which occurs from repeated cyclic loading, where the resulting stresses in the bone are below the yield stress, rather than a fracture that occurs after a single traumatic load. It is a common overuse injury among running athletes, ballet dancers, and military recruits (Milgrom et al. Citation1985; Armstrong et al. Citation2004). In a study of 91 patients with stress fractures, 51 were located in the tibia. (Milgrom et al. Citation1985) Stress fractures at the anterior border of the tibial diaphysis heal very slowly, or not at all (Burrows Citation1956). Hence, they have ended the careers of many top athletes. Treatment by rest alone allows less than half of the athletes to return to sports (Beals & Cook Citation1991). Therefore, surgical treatment is normally advocated.
The surgical treatment usually aims at providing complete stability, so that the mechanical function of the bone at the lesion is taken over by an implant (Chang & Harris Citation1996; Varner et al. Citation2005; Gregory & Guyton Citation2006; Liimatainen et al. Citation2009). One study reports favorable outcome of plating at the lateral side of the tibia, for which the treatment was successful in 28 out of 29 patients (Liimatainen et al. Citation2009). Intramedullary nailing has been successful in several studies (Chang & Harris Citation1996; Varner et al. Citation2005; Gregory & Guyton Citation2006). These surgical methods are rather invasive, with a considerable risk of associated morbidity, and a need for a secondary surgery to remove the implant. Moreover, one study has reported the outcome of transversal drilling of the stress fracture (Miyamoto et al. Citation2009). In a series of five elite dancers, several small transversal holes (2.0 mm diameter) were drilled through the crack and into the medullary cavity. In addition, bone grafts were used. All patients returned to dance after an average of 5.5 months post-operation. The improved healing was most likely a result of the combined new surgical trauma and the addition of bone graft.
A possible explanation for the low healing capacity of these fractures can be found in the different tissue differentiation theories that have been proposed (Perren & Cordey Citation1980; Prendergast et al. Citation1997; Carter & Beauprt Citation1998; Claes & Heigele Citation1999; Isaksson, Donkelaar et al. Citation2006). One example is the theory of fracture gap strain in the context of plate fixation of ordinary fractures, described by Perren and Cordey (Citation1980) and Perren (Citation1979). They proposed that for any tissue to form between two fracture surfaces, the strain in the gap tissue must not exceed the rupture limit of that tissue. In addition, they suggested limits for different tissue types, with a tissue strain limit for bone around 2%, and with the highest possible strain for any tissue other than granulation tissue around 15%. In most bone fractures, these threshold values are seldom exceeded, because the fracture gaps are wide in relation to the displacement that occurs. However, with a very thin fracture gap, as in a stress fracture, small (sub-millimeter) dislocations of the crack will lead to large strains. We therefore hypothesized that the deformation of the tibia during physiological loading would cause strains in the soft tissue in a transverse crack at the anterior border that prohibit cell survival. If so, a rational treatment would be to widen the fracture gap and thus reduce the strains below the threshold values. This may be done by drilling one wide hole, perpendicular to the bone surface.
The purpose of the present study was to investigate the biomechanical effects of such a treatment by developing a three-dimensional finite element (FE) model of a human tibia. Hence, we aim to evaluate the stress–strain state in the tibia with a stress fracture and in the tibia with an enlarged stress fracture (drilled hole), with focus on the soft tissue in the crack that is responsible for the healing, to understand the effect of the potential drilling surgery on the tissue state. Finally, we aim to evaluate the effects of the potential drilling surgery on the overall stiffness of the tibia.
Materials and methods
Clinical images and geometry
The geometry of the tibia was acquired from clinical CT images (Somatom Definition, Siemens AG, Erlangen, Germany; voxel size 0.6 × 0.6 × 0.5 mm) of a 43-year-old female with no defects or skeletal diseases reported in the right tibia. The inner and outer surfaces of the cortical bone were extracted as triangulated surfaces using a semi-automatic threshold algorithm (Yushkevich et al. Citation2006). Using an automatic reverse engineering algorithm (Rhino v4.0, Robert McNeel & Associates, Seattle, USA), non-uniform rational B-splines (NURBS) surfaces were created from the triangulated surfaces. From these images, three geometries were developed: (1) an intact tibia, (2) a tibia with a stress fracture, and (3) a tibia with a stress fracture which also had undergone drilling surgery (Figure ). A reference system based on palpation of anatomical landmarks was used to define axes in the anterior and medial directions, and in the direction of the long bone (Ruff & Hayes Citation1983).
Figure 1. Geometries for the computational models. Overview of the intact tibia (left), and magnifications of the intact (top), stress fractured (middle), and drilled (bottom) tibia. Light gray elements are cortical bone, whereas dark gray elements are granulation tissue.
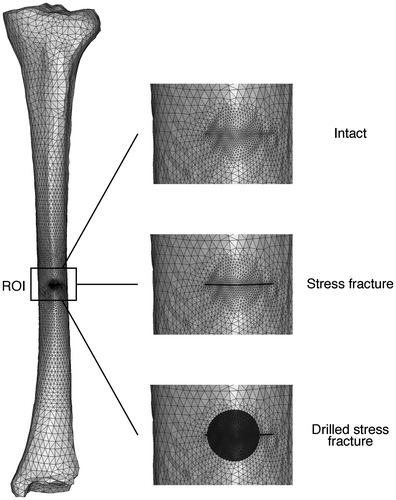
The location of the stress fracture at the long bone axis and its depth (4.0 mm) were measured from computed tomography images and clinical radiographs in the sagittal plane of a 25-year-old male suffering from a stress fracture at the anterior border of the tibial diaphysis. The location of the stress fracture was linearly scaled to the biomechanical length. The available medical report of the subject indicated that the stress fracture started at the anterior border of the tibia, and extended towards the centroid of the transversal cross section. The normal distance between the crack surfaces was estimated to be 0.2 mm, based on histological studies of stress fractures (Aspenberg et al. Citation2010).
The potential drilling surgery was mimicked by creating a 7.4 mm diameter hole beginning at the anterior border, through the crack, and into the medullary cavity. The stress fracture was bigger than the diameter of the hole, leaving the remaining of the crack at the sides of the hole (Figure ). Only cortical bone was removed, and the marrow was left intact. For modeling purposes, the hole was assumed to fill up with granulation tissue. Patient images came from subjects participating in a clinical study of stress fractures with ethical approval from the Regional Ethics Board in Östergötland (# M81-07, T13-08).
Finite element modeling
All models were based on a versatile three-dimensional mesh created using the NURBS surfaces of the bone (Hypermesh v11.0, Altair Engineering, Inc., Troy, USA). All simulations were conducted in ABAQUS (v6.9-EF1, Dassault Systemes HQ, Vélizy-Villacoublay, France). Tetrahedral 10-noded elements with modified displacement formulation and linear pore pressure were used (C3D10MPH Abaqus element type). The mesh contained a total of ~173 000 elements, and had an average element volume of 1.6 mm³. A convergence test was made to ensure that a suitable mesh density was used. The total strain energy was calculated for consecutively finer meshes. The mesh was considered accurate enough when its strain energy was within ±5.0% of that of the reference mesh. All load cases were ramped from zero to full load during a time period of 0.5 s.
The tibia was assumed to be poroelastic with material properties according to Table . Large displacements were assumed by enabling the NLGEOM option, and therefore logarithmic strains were calculated. The diaphyseal cortical bone was assumed to be orthotropic, with its principal axis in the direction of the long axis. The epiphyseal cortical bone, trabecular bone, bone marrow and granulation tissue were assumed to be isotropic. Element-specific mapping of Young’s modulus based on radiological density of the CT data was performed for the trabecular bone (Linde et al. Citation1992). The external surfaces of the cortical bone and granulation tissue were assumed to be impermeable (Einhorn Citation1998; Gardinier et al. Citation2010).
Table 1. Material properties. L – Longitudinal direction; T – transversal directions; L–T – longitudinal–transversal plane; T–T transversal–transversal plane. All material properties for bone marrow and granulation tissue were assumed based on previous computational studies. (Isaksson, Donkelaar et al. Citation2006; Isaksson, Wilson et al. Citation2006; Isaksson et al. Citation2009).
Validation of stiffness of intact tibia
For validation of the FE mesh and material description, the stiffness of the intact tibia was evaluated and compared to in vitro tests by Cristofolini et al. (Citation2010). Boundary conditions from the experiment were mimicked to simulate four-point bending and torsional testing. Bending stiffness was defined as the ratio between the applied load and the deflection in a point equidistant from the two inner-most points in a four point bending rig. Similarly, torsional stiffness was defined as the ratio between the applied torque and the maximum rotational displacement of the proximal epiphysis around the long axis. In the validation of the stiffness of the model, a bending rig with 58 mm spacing between the points was modeled. A range of material properties and material models were collected from literature, and the final set described above was chosen based on an extensive parameter study (Fågelberg Citation2012).
Effect of drilling on the overall tibia stiffness
For evaluating how the overall stiffness of the tibia is affected by the crack and drilling surgery, bending stiffness and torsional stiffness were evaluated. A four-point bending test was simulated with all three models, using a bending rig with 80 mm spacing. Torsional stiffness was evaluated as described for the validation of the tibial stiffness against in vitro data.
Stress and strain in the tibia and fracture gap
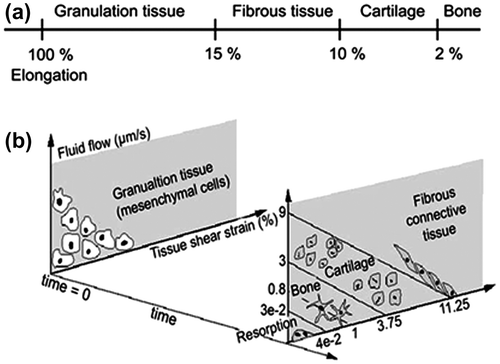
For evaluation of the resulting spatial stress and strain distributions as a result of a stress fracture and the potential drilling surgery, two different loading conditions were simulated. Loading conditions representative of gait and stair climbing were obtained from the literature (Kutzner et al. Citation2010). Only the tibio-femoral bone-to-bone contact force was used, and the highest load during the activity cycle was identified for each load case. The load distribution was shared between the medial (60%) and the lateral condyle (40%) (Morrison Citation1970). The forces for each activity are presented in Table . The distal end was fixed to prevent rigid body motion. The state of stress in the tibia was evaluated in terms of von Mises stress, whereas the state of strain was evaluated in terms of major and minor principal strains. These were used as an indication of whether the crack will propagate or not. The states of strain in the fracture gap were evaluated using major principal strain and deviatoric strain. Moreover, the fluid velocity was evaluated. These stimuli were chosen since they have been suggested to guide the tissue differentiation process (Perren & Cordey Citation1980; Prendergast et al. Citation1997). The importance of the predicted mechanical stimulation was evaluated by comparing the stimulation outcome with the thresholds from the tissue differentiation schemes proposed by Perren and Cordey (Citation1980) and Prendergast et al. (Citation1997) (Figure ). The latter algorithm is based on biphasic theory, where the magnitudes of deviatoric strain and interstitial fluid velocity are combined to indicate the type of tissue that is formed spatially. The thresholds were originally proposed by comparing the simulation with data from tissue ingrowth onto an orthopedic implant (Huiskes et al. Citation1997). In earlier studies, it has for example successfully predicted adaptive bone formation around implants, in bone chambers, and during fracture healing (Isaksson Citation2012; Khayyeri et al. Citation2015). In the current study, both algorithms were implemented in a non-adaptive fashion, where the outcome of the simulations were compared to the thresholds in Figure .
Table 2. Tibio-femoral contact force as percentage of body weight (Kutzner et al. Citation2010). In this study, a body weight of 60 kg was assumed.
Figure 2. Mechano-regulatory algorithms. (a) Perren and Cordey’s idea based on how much elongation each tissue type can tolerate (Perren & Cordey Citation1980). (b) The tissue differentiation scheme proposed by Prendergast et al. based on the magnitudes of fluid velocity and tissue shear strain (Prendergast et al. Citation1997).
Results
Validation of stiffness of intact tibia
The stiffness of the intact tibia model was within the range of the experimental data (Cristofolini et al. Citation2010). In our model, the torsional stiffness was 7% higher, and the bending stiffness in the coronal and sagittal plane were 0.3% lower and 16% higher than the average stiffness reported, respectively. This is well within the experimental variability between patients.
Effect of drilling on overall tibia stiffness
When comparing all three models (intact tibia, tibia with stress fracture, and tibia with drilled stress fracture), the drilling was found to have a minor effect on the overall stiffness of the tibia. The largest difference was noted for bending in the sagittal plane, in which a decrease of 9.9% was predicted compared to the intact tibia, whereas the tibia with a stress fracture was 2.6% less stiff than the intact tibia. The drilled tibia was predicted to be 1.3% less stiff than the intact tibia in torsion, whereas the tibia with a stress fracture was 0.1% less stiff.
Strain and stress in the tibia
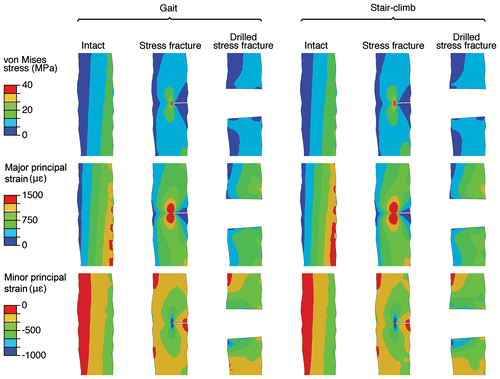
All models and load cases predicted the anteromedial side of the tibia to be in tension, whereas the posterolateral side was in compression. Overall, in the intact tibia, maximum major principal strains of 2200 με were predicted at the anterior side of the bone surface during gait, which increased to 2500 με during stair climbing. For the minimum of the minor principal strain, magnitudes of –2900 and –3200 με were predicted at the posterior surface of the diaphysis during gait and stair climbing, respectively. The models predicted an increase of the minimum principal strains at the posterior diaphysis as a result of drilling. The direction of the principal strains at the anterior and posterior diaphysis coincided in general with the long axis. In the intact tibia during gait, the maximum magnitude of the von Mises stress was 42 MPa, which was predicted in the posterior surface of the diaphysis. During stair climbing, the maximum von Mises stress increased to 47 MPa at the same location.
When viewing the sagittal plane through the fracture gap, the intact tibia model predicted maximum von Mises stresses of 22 and 27 MPa during gait and stair climbing in the anterior part, respectively (Figure ). The model with a stress fracture predicted lower stresses in the cross section, however, stress concentrations were apparent at the crack tip (Figure ). The model with a drilled stress fracture predicted lower stresses than the model of the intact tibia, with no stress concentrations in the drilled hole (Figure ). Both the predicted stress and strain states in the bone and close to the crack tip were within the linear elastic region of bone (Bayraktar et al. Citation2004; Ohman et al. Citation2008). Thus, no additional damage or crack growth is expected with these loading conditions. However, to evaluate this further at the absolute crack tip, a fracture mechanics approach is needed.
Figure 3. Strain and stress fields in the cortical bone during gait and stair climbing. The images are displayed as sagittal cross sections in the cortical bone at the lesion of the intact, stress fractured and drilled tibia during gait and stair climb. The granulation tissue is removed for clarity.
Stress and strain in the soft tissue in the fracture gap
During gait, the model of the tibia with a stress fracture predicted major principal strains greater than 10% in the larger portion of the granulation tissue in the fracture gap (maximum 35%), whereas deviatoric strains greater than 8% were predicted in most of the same region (Figure ). The magnitudes of the fluid velocity were predicted to be 1–6 μm/s at the external boundaries of the granulation tissue (Figure ). Similar results were predicted during stair climbing, but with slightly higher magnitudes of the major principal strain (11%) and deviatoric strain (9%) for most of the granulation tissue. The maximum fluid velocity increased to 6.5 μm/s (Figure ).
Figure 4. Strain fields and fluid velocity in the granulation tissue during gait and stair climbing. Major principal strain, fluid velocity and deviatoric shear strain in the granulation tissue of the fracture gap of a stress fracture and a drilled stress fracture are displayed. Only the elements inside the soft tissue (crack, or crack plus hole) are shown, looking from the anterior side of the tibia into the bone, crack and hole.
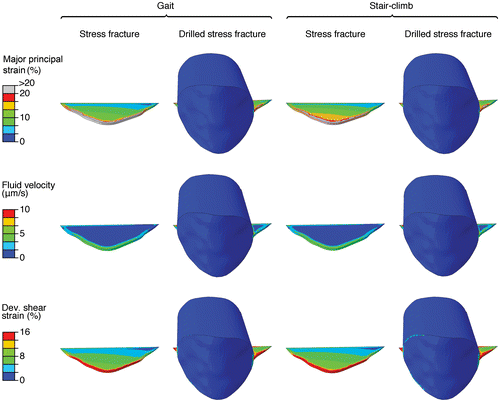
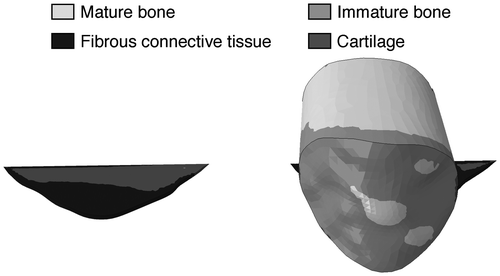
After drilling, the magnitudes of the major principal strain and deviatoric strain decreased to less than 1% during both gait and stair climbing for most of the granulation tissue (Figure ). Fluid velocities of less than 0.5 μm/s were predicted in the drilled hole (Figure ). To put the magnitudes of the mechanical stimulation in perspective, the suggested tissue differentiation thresholds in the algorithms by Perren and Cordey (Citation1980) and Prendergast et al. (Citation1997) was implemented (without adaptation). The findings of both theories were consistent (Figure ). According to the differentiation theory by Prendergast, stair climbing would result in mainly fibrous tissue in the stress fracture, with some cartilage formation at the innermost crack tip (Figure ). The significant decrease of the interfragmentary strains predicted by drilling would instead stimulate formation of stiffer tissues such as immature and mature bone (Figure ).
Figure 5. The fraction of elements in the crack and the drilled hole predicted to become each of the respective tissue types with the algorithms by (A) Perren and Cordey (Citation1980) and (B) Prendergast et al. (Citation1997).
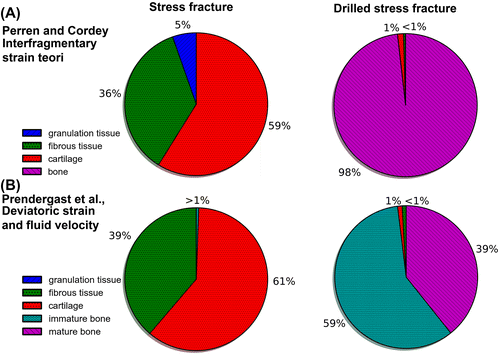
Figure 6. The predicted tissue differentiation during stair climb according to the mechano-regulation algorithm by Prendergast et al. (Citation1997) based on the magnitudes of fluid velocity and deviatoric shear strain.
Discussion
The purpose of this study was to evaluate the biomechanical effects of transversal drilling of a stress fracture at the anterior border of the tibia, with focus on the soft granulation tissue in the crack gap. We found a large decrease of the strains in the granulation tissue filling the fracture gap, with only a minor decrease in the overall stiffness of the tibia as a result of drilling. Our hypothesis that the strains in the fracture gap before drilling would be large enough to prohibit bone formation is supported by the results, as well as the decrease of interfragmentary strain after drilling.
Although stress fractures at the anterior border of the tibia have been subject for earlier FE studies, (Sonoda et al. Citation2003; Wong et al. Citation2010) no biomechanical explanation for the low healing capacity has been proposed. The change in strain levels was significant, and comparison with mechano-regulatory theories such as the interfragmentary strain theory by Perren and Cordey (Citation1980) and the tissue differentiation scheme by Prendergast et al. (Citation1997) supported the findings. To the authors’ knowledge, the present study is the first to predict strains and fluid velocities in the fracture gap of a stress fracture. The available experimental data regarding material parameters for soft tissues are highly limited. Therefore, those properties were assumed based on previous computational studies using poroelastic models (Isaksson, Donkelaar et al. Citation2006; Isaksson, Wilson et al. Citation2006; Isaksson et al. Citation2009).
Stress concentrations at the tip of the crack were observed in the models with a stress fracture and with a drilled stress fracture (Figure ). If the diameter of the hole is increased to such an extent that the entire crack is removed, these stress concentrations would not be apparent, and the risk of crack propagation would be nonexistent in the drilled tibia. However, a smaller diameter was chosen, since some patients may suffer from a stress fracture that is too large to remove completely. Moreover, in the clinical setting the crack propagation is limited by the pain the patient is suffering. Therefore, the aim of drilling would not be to stop crack propagation, but to facilitate healing.
Large strains were predicted in the fracture gap before the drilling surgery. Hence, tissue differentiation theories may explain the low healing capacity of stress fractures. Using the load case with the largest strains (i.e. stair climbing), and according to the differentiation theory by Prendergast et al. (Citation1997), the outermost half of the granulation tissue would differentiate into fibrous tissue, whereas the innermost crack tip will differentiate into cartilage (Figure ). A significant decrease of the interfragmentary strains was predicted after the fracture gap had been drilled. Based on both tissue differentiation theories, such change is believed to stimulate formation of stiffer tissues, and this is supported by the results which predicts mature bone formation in a large portion of the fracture gap (Figure ). Some immature bone is predicted in the outermost (periosteal) part of the granulation tissue (Figure ). The question arises whether the predicted increase of stiffness in the untreated fracture gap is enough to eventually allow formation of bone. Thus, use of computational modeling to investigate the healing process in the soft tissue in the gap over time may provide valuable information in the development of new surgical methods for treating stress fractures at the anterior border of the tibial diaphysis. Current surgical methods (e.g. plating and intramedullary nailing) concur with the tissue differentiation theories in that they aim at providing complete stability of the fracture gap, thus reducing interfragmentary strains.
There is very limited data on the histology of stress fractures in cortical bone. A case report of a stress fracture in the femoral cortex of an elderly person showed a fracture gap of less than 0.2 mm, with a complete absence of living cells (Aspenberg et al. Citation2010). The crack contained only necrotic material. However, this patient had a so-called atypical fracture, i.e. a stress fracture associated with bisphosphonate medication, which is likely to inhibit the ability to stop crack growth via local targeted removal and replacement of the bone. This histological picture has been noted in other similar cases, and also in a patient who got a femoral cortical stress fracture without taking bisphosphonates (Schilcher et al. Citation2014). A fibrous tissue is often noted at the outer surface of the bone where the crack reaches the surface, but this tissue is obviously unable to grow into the crack. These histological observations support the assumptions regarding the dimensions of the fracture gap in this study, but it seems that, at least in these cases, the mechanical environment in the crack was incompatible with survival of any tissue at all.
A limitation of the present study is that only the tibio-femoral contact force was simulated. Although being common in FE studies of long bones (Duda et al. Citation2001; Sonoda et al. Citation2003; Trabelsi et al. Citation2011; Son & Chang Citation2013), this assumption might lead to an overestimation of the bending state in long bones (Duda et al. Citation2001; Speirs et al. Citation2007). However, in this study, we have only simulated daily activities such as walking and stair climbing. Hence, the strains during athletic activities will be significantly higher. Ideally, a complete description of the muscle forces would be favorable, as it could more accurately describe the strain state underwent by the bone (Duda et al. Citation1998; Polgar et al. Citation2003; Phillips Citation2009). However, the obtained magnitudes of the diaphyseal strains during gait in this study are of the same order of magnitude as those reported for two discrete points at the medial side in in vivo experiments (Ekenman et al. Citation1998; Ekenman et al. Citation2002). We chose to model the external surface of the granulation tissue in the drilled hole as impermeable with the motivation that the fashia covering the bone is more or less impermeable (Einhorn Citation1998). This assumption may be questionable in the case of the drilled hole. However, we have performed the simulations also with the assumption of 0 pore pressure (free flow) at the external surface of the crack and hole. The free-flow simulations obviously results in higher fluid velocities (roughly a factor 10) on the surface (first few elements). However, it does not affect the outcome of our study in terms of the strain environment, and it does not change the prediction with either of the mechano-regulatory theories. Furthermore, the shape and dimensions of the crack are based on clinical experience and radiological images from one patient. By investigating more patients, the influence of the crack size and geometry, as well as the hole diameter, a more complete characterization of the underlying mechanical processes could be accomplished. For example, micro-CT scans of biopsies could be used to increase the accuracy of the crack geometry.
This study focused on characterizing the mechanical environment in a stress fracture and the mechanical consequences of drilling a hole around the stress fracture. Bone healing is known to be largely driven by mechanical factors (Kenwright et al. Citation1991; Claes et al. Citation1998), and even though we show that this procedure may be beneficial from a mechanical perspective, it is important to emphasize that the drilling will also affect the biological milieu. It will generate a new trauma followed by an inflammatory phase where growth factors, angiogeneic factors and mesenchymal stem cells will be recruited to the area to initiate the healing response (Einhorn Citation1998). The effects of these factors are however outside the scope of the current study and need to be addressed in experimental or clinical studies.
With the aim to promote healing in the established crack, we chose to investigate the potential of one wide drilled hole through the crack. The reason for drilling one hole instead of several smaller holes (Liimatainen et al. Citation2009) was that it is quite difficult to exactly identify the crack using pre-operative radiography. It is easy to see the localized cortical thickening, but this can be one–two centimeters wide, and the crack is hidden somewhere in it. Therefore, it is technically very challenging to hit the crack with small drill holes. Moreover, the local reduction in strain stimuli will be less in smaller holes.
The large interfragmentary strains provide a biomechanical explanation for the low healing rate of stress fractures at the anterior border of the tibial diaphysis. An increase of the fracture gap reduces the strains to a level where bone formation may be possible. Therefore, transversal drilling, using a drill with a large diameter, may be considered for clinical testing as treatment of these fractures.
The poor healing capacity of anterior tibial stress fractures has been an enigma, to which the present simulation provides a reasonable answer. Moreover, based on the simulation results, we propose a surgical treatment principle, which might allow these fractures to heal without an increased risk of dislocation and without the need for implants or large surgical exposures. The healing might be expected to be reasonably fast (Miyamoto et al. Citation2009). We would suggest those institutions that have sufficient numbers of stress fracture patients to try the principle, preferably in a randomized, controlled fashion.
Conflict of interest disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Armstrong DW, Rue JP, Wilckens JH, Frassica FJ. 2004. Stress fracture injury in young military men and women. Bone. 35:806–816.10.1016/j.bone.2004.05.014
- Ashman RB, Rho JY. 1988. Elastic modulus of trabecular bone material. J Biomech. 21:177–181.10.1016/0021-9290(88)90167-4
- Aspenberg P, Schilcher J, Fahlgren A. Histology of an undisplaced femoral fatigue fracture in association with bisphosphonate treatment. Frozen bone with remodelling at the crack. Acta Orthop Scand. 2010;81:460–462. 10.3109/17453674.2010.492766
- Bayraktar HH, Morgan EF, Niebur GL, Morris GE, Wong EK, Keaveny TM. 2004. Comparison of the elastic and yield properties of human femoral trabecular and cortical bone tissue. J Biomech. 37:27–35. doi:10.1016/S0021-9290(03)00257-4.
- Beals RK, Cook RD. 1991. Stress fractures of the anterior diaphysis. Orthopaedics. 14:869–875.
- Burrows HJ. 1956. Fatigue infraction of the middle of the tibia in ballet dancers. J Bone Joint Surg. 38B:83–94.
- Carter DR, Beauprt GS. 1998. Mechanobiology of skeletal regeneration. Clin Orthop. 355:41–55.10.1097/00003086-199810001-00006
- Chang PS, Harris RM. 1996. Intramedullary nailing for chronic tibial stress fractures: a review of five cases. Am J Sports Med. 24:688–692.10.1177/036354659602400522
- Claes LE, Heigele CA. 1999. Magnitudes of local stress and strain along bony surfaces predict the course and type of fracture healing. J Biomech. 32:255–266.10.1016/S0021-9290(98)00153-5
- Claes LE, Heigele CA, Neidlinger-Wilke C, Kaspar D, Seidl W, Margevicius KJ, Augat P. 1998. Effects of mechanical factors on the fracture healing process. Clin Orthop Relat Res. 355:S132–47.10.1097/00003086-199810001-00015
- Cowin SC. 1999. Bone poroelasticity. J Biomech. 32:217–238.10.1016/S0021-9290(98)00161-4
- Cristofolini L, Conti G, Juszczyk M, Cremonini S, Van Sint Jan S, Viceconti M. 2010. Structural behaviour and strain distribution of the long bones of the human lower limbs. J Biomech. 43:826–835.10.1016/j.jbiomech.2009.11.022
- Duda GN, Heller M, Albinger J, Schulz O, Schneider E, Claes L. 1998. Influence of muscle forces on femoral strain distribution. J Biomech. 31:841–846.10.1016/S0021-9290(98)00080-3
- Duda GN, Mandruzzato F, Heller M. 2001. Mechanical boundary conditions of fracture healing: borderline indications in the treatment of unreamed tibial nailing. J Biomech. 34:639–650.10.1016/S0021-9290(00)00237-2
- Einhorn TA. 1998. The cell and molecular biology of fracture healing. Clin Orthop. 355:7–21.10.1097/00003086-199810001-00003
- Ekenman I, Halvorsen K, Westblad P, Felländer-Tsai L, Rolf C. 1998. Local bone deformation at two predominant sites for stress fractures of the tibia: an in vivo study. Foot Ankle Int. 19:479–484.10.1177/107110079801900711
- Ekenman I, Milgrom C, Finestone A, Begin M, Olin C, Arndt T, Burr D. 2002. The role of biomechanical shoe orthoses in tibial stress fracture prevention. Am J Sports Med. 30:866–870.
- Fågelberg E. Mechanical analysis of tibial stress fractures. A finite element study investigating the potential of a new surgical treatment [MSc thesis]. Lund: Lund University. 2012:1–72.
- Gardinier JD, Townend CW, Jen K-P, Wu Q, Duncan RL, Wang L. In situ permeability measurement of the mammalian lacunar-canalicular system. Bone. 2010;46:1075–1081. 10.1016/j.bone.2010.01.371
- Gregory P, Guyton MD. 2006. Intramedullary nailing of tibial stress fractures. Oper Tech Sports Med. 14:259–264.
- Huiskes R, Van Driel WD, Prendergast PJ, Soballe K. 1997. A biomechanical regulatory model for periprosthetic fibrous-tissue differentiation. J Mater Sci Mater Med. 8:785–788.10.1023/A:1018520914512
- Isaksson H. Recent advances in mechanobiological modeling of bone regeneration. Mech Res Commun. 2012;42:22–31. 10.1016/j.mechrescom.2011.11.006
- Isaksson H, Donkelaar CCV, Huiskes R, Ito K. Corroboration of mechanoregulatory algorithms for tissue differentiation during fracture healing: comparison with in vivo results. J Orthop Res. 2006:898–907.10.1002/jor.20118
- Isaksson H, Wilson W, van Donkellar CC, Huiskes R, Ito K. 2006. Comparison of biophysical stimuli for mechano-regulation of tissue differentiation during fracture healing. J Biomech. 39:1507–1516.10.1016/j.jbiomech.2005.01.037
- Isaksson H, van Donkelaar CC, Ito K. Sensitivity of tissue differentiation and bone healing predictions to tissue properties. J Biomech. 2009;42:555–564. 10.1016/j.jbiomech.2009.01.001
- Johnson MW, Chakkalakal DA, Harper RA, Katz JL, Rouhana SW. 1982. Fluid flow in bone in vitro. J Biomech. 15:881–885.10.1016/0021-9290(82)90054-9
- Kenwright J, Richardson JB, Cunningham JL, White SH, Goodship AE, Adams MA, Magnussen PA, Newman JH. Axial movement and tibial fractures. A controlled randomised trial of treatment. J Bone Joint Surg Br. 1991;73:654–659.
- Khayyeri H, Isaksson H, Prendergast PJ. 2015. Corroboration of computational models for mechanoregulated stem cell differentiation. Comput Methods Biomech Biomed Eng. 18:15–23. doi:10.1080/10255842.2013.774381.
- Kutzner I, Heinlein B, Graichen F, Bender A, Rohlmann A, Halder A, Beier A, Bergmann G. 2010. Loading of the knee joint during activities of daily living measured in vivo in five subjects. J Biomech. 43:2164–2173.10.1016/j.jbiomech.2010.03.046
- Liimatainen E, Sarimo J, Hulkko A, Ranne J, Heikkilä J, Orava S. 2009. Anterior mid-tibial stress fractures. Results of surgical treatment. Scand J Surg. 98:244–249.
- Linde F, Hvid I, Madsen F. The effect of specimen geometry on the mechanical behaviour of trabecular bone specimens. J Biomech. 1992;25:359–368. Available from: http://www.ncbi.nlm.nih.gov/pubmed/1583015 10.1016/0021-9290(92)90255-Y
- Milgrom C, Giladi M, Stein M, Kashtan H, Margulies JY, Chisin R, Steinberg R, Aharonson Z. 1985. Stress fractures in military recruits. A prospective study showing an unusually high incidence. J Bone Joint Surg. 67:732–735.
- Miyamoto RG, Dhotar HS, Rose DJ, Egol K. 2009. Surgical treatment of refractory tibial stress fractures in elite dancers: a case series. Am J Sport Med. 37:1150–1154.10.1177/0363546508330973
- Morrison JB. 1970. The mechanics of the knee joint in relation to normal walking. J Biomech. 3:51–61.10.1016/0021-9290(70)90050-3
- Ohman C, Dall’Ara E, Baleani M, Van Sint Jan S, Viceconti M. The effects of embalming using a 4% formalin solution on the compressive mechanical properties of human cortical bone. Clin Biomech (Bristol, Avon). 2008;23:1294–1298. doi:10.1016/j.clinbiomech.2008.07.007. 10.1016/j.clinbiomech.2008.07.007
- Perren S. Physical and biological aspects of fracture healing with special reference to internal fixation. Clin Orthop Relat Res. 1979:175–196.
- Perren SM, Cordey J. 1980. The concept of interfragmentary strain. In: Uhthoff HK, editor. Current concepts of internal fixation of fractures. Berlin: Springer-Verlag; p. 63–77.
- Phillips ATM. 2009. The femur as a musculo-skeletal construct: a free boundary condition modelling approach. Med Eng Phys. 31:673–680. doi:10.1016/j.medengphy.2008.12.008.
- Polgar K, Gill HS, Viceconti M, Murray DW, O’Connor JJ. 2003. Strain distribution within the human femur due to physiological and simplified loading: finite element analysis using the muscle standardized femur model. Proc Inst Mech Eng H. 217:173–189.10.1243/095441103765212677
- Prendergast P, Huiskes R, Søballe K. 1997. Biophysical stimuli on cells during tissue differentiation at implant interfaces. J Biomech. 30:539–548.10.1016/S0021-9290(96)00140-6
- Ruff CB, Hayes WC. Cross-sectional geometry of Pecos Pueblo femora and tibiae – a biomechanical investigation: II. Sex, age, and side differences. Am J Phys Anthropol. 1983;60:383–400.10.1002/(ISSN)1096-8644
- Schaffler MB, Burr DB. 1988. Stiffness of compact bone: effects of porosity and density. J Biomech. 21:13–16.10.1016/0021-9290(88)90186-8
- Schilcher J, Sandberg O, Isaksson H, Aspenberg P. 2014. Histology of 8 atypical femoral fractures: remodeling but no healing. Acta Orthop. 85:280–286. doi:10.3109/17453674.2014.916488.
- Smit TH, Huyghe JM, Cowin SC. 2002. Estimation of the poroelastic parameters of cortical bone. J Biomech. 35:829–835.10.1016/S0021-9290(02)00021-0
- Son D-S, Chang S-H. 2013. The simulation of bone healing process of fractured tibia applied with composite bone plates according to the diaphyseal oblique angle and plate modulus. Compos Part B Eng. 45:1325–1335.10.1016/j.compositesb.2012.07.037
- Sonoda N, Chosa E, Totoribe K, Tajima N. 2003. Biomechanical analysis for stress fractures of the anterior middle third of the tibia in athletes: nonlinear analysis using a three-dimensional finite element method. J Orthop Sci. 8:505–513.10.1007/s00776-003-0671-5
- Speirs AD, Heller MO, Duda GN, Taylor WR. 2007. Physiologically based boundary conditions in finite element modelling. J Biomech. 40:2318–2323.10.1016/j.jbiomech.2006.10.038
- Trabelsi N, Yosibash Z, Wutte C, Augat P, Eberle S. 2011. Patient-specific finite element analysis of the human femur – a double-blinded biomechanical validation. J Biomech. 44:1666–1672.10.1016/j.jbiomech.2011.03.024
- Varner KE, Younas SA, Lintner DM, Marymont JV. Chronic anterior midtibial stress fractures in athletes treated with reamed intramedullary nailing. Am J Sports Med. 2005;33:1071–1076.10.1177/0363546504271968
- Williams J. Ultrasonic wave propagation in cancellous and cortical bone: prediction of some experimental results by Biot’s theory. J Acoust Soc Am. 1992;91:1106–1112. 10.1121/1.402637
- Wong C, Mikkelsen P, Hansen LB, Darvann T, Gebuhr P. 2010. Finite element analysis of tibial fractures. Dan Med Bull. 57:1–4.
- Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. 2006. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 31:1116–1128.10.1016/j.neuroimage.2006.01.015
