Abstract
Geometric characteristics of a vascular anastomosis can have important biomechanical impacts, particularly in the hemodynamic context of wall shear stress (WSS). In this study, we propose a new branching anastomosis design, the Hybrid anastomosis, to connect a donor vessel to two recipient vessels. The central obstruction characteristic of the traditional Figure 8 anastomosis is mitigated by creating a recessed lumen at the bifurcation. Chicken vessels were used to create and test three Figure 8 and three Hybrid anastomoses ex vivo. Computational fluid dynamics (CFD) simulations of idealized anastomosis designs were then used to verify the experiments and further studied the WSS and WSS gradient (WSSG) profiles of the designs. Experimental results showed increased flow through the Hybrid anastomosis (0.40 ml/s vs. 0.23 ml/s). The mean suture time averaged 20 and 28 min for the Figure 8 and Hybrid anastomoses, respectively. CFD simulations agreed with the experiments; the simulations showed lower flow resistance in the Hybrid anastomosis (1.89 kPa-s/ml vs. 6.77 kPa-s/ml). Most importantly, a set of more physiologically realistic simulations showed that the Hybrid anastomosis reduced WSS and WSSG by over 60% as compared to the Figure 8. Results demonstrated that the proposed Hybrid anastomosis can be created on a comparable surgical time scale (and from the same initial vasculature) as compared to the Figure 8 anastomosis, while decreasing flow resistance and reducing WSS and WSSG considerably. These findings support that the new Hybrid anastomosis may represent a favorable surgical alternative to the Figure 8 design.
Introduction
The anastomosis of blood vessels is one of the most fundamental vascular surgical procedures. A surgical anastomosis is necessary whenever blood vessels need to be connected together. One class of examples includes when a part of a blood vessel is diseased and the only way to restore normal flow is by removing that part and then reconnecting the healthy vasculature. Arterial bypass of stenotic coronary arteries, cerebral aneurysm and brain tumor resection, cerebral infarction treatment, portocaval shunting, and treatment of Mayamoya disease are several specific examples of procedures that may call for surgical anastomoses (Peerless & Mervart Citation1984; Ojha Citation1993; Tzakis et al. Citation1993; Okada et al. Citation1998). Anastomosis geometry is known to impact local hemodynamics and may lead to vascular remodeling (Lasheras Citation2007; Sousa et al. Citation2012). Disturbed flow patterns caused by geometric transitions at the anastomosis site (as well as altered biomechanical properties of the blood vessel walls themselves) may lead to intimal hyperplasia, atherosclerosis, and/or platelet thrombosis (Ojha Citation1993; Archie et al. Citation2001; Cunningham & Gotlieb Citation2005; Loth et al. Citation2008). High wall shear stress (WSS) and wall shear stress gradient (WSSG) are two factors proposed to underlie such outcomes (Ku Citation1997; Lei et al. Citation1997). Therefore, it is essential to design anastomoses that restore normal circulation without effecting high WSS or WSSG.
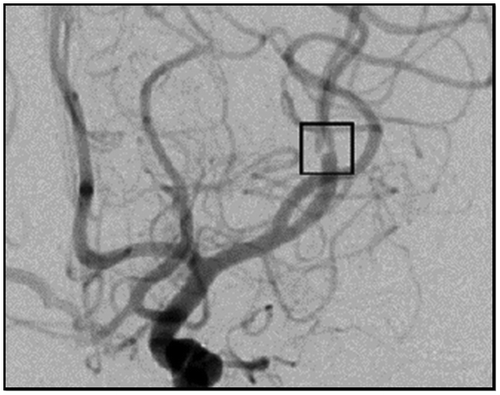
In a previously published case report, we presented the anastomosis of a single donor artery to two smaller recipient arteries after excision of a large intracranial aneurysm, where two branches of the middle cerebral artery (MCA) arose from the aneurysmal dome (Hanel & Spetzler Citation2008). After excision of the aneurysm, the two small recipient vessels were anastomosed to the larger M1 MCA using a Figure 8 anastomosis. The anastomosis procedure was used to recreate the MCA bifurcation in this case, but generalizes well to the connection of any larger donor vessel to two smaller recipients. In a postoperative angiographic view of the anastomosis a translucency was observed at the anastomosis site (shown in the center of the small square within Figure ) where a central obstruction was created by the double thickness of the vessel wall. The presence of any luminal abnormality may increase WSSG within the anastomosis thereby promoting vascular remodeling.
Figure 1. Post-operative anterior/posterior angiographic view of a Figure 8 anastomosis. The translucency at the center of the black box represents the obstruction created by the two vessel walls at the middle of the anastomosis. Used with permission from Barrow Neurological Institute.
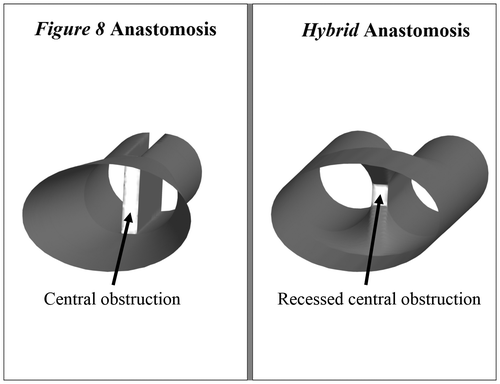
To compensate for the central obstruction characteristic of the Figure 8 anastomosis design, we designed a new Hybrid anastomosis with a recessed lumen. The new anastomosis design is created by making a simple cut on the inner surface of the recipient vessels, and then suturing each half of the cut to the opposite vessel, rather than suturing the two vessels at the midline. The Hybrid design was tested experimentally using ex vivo chicken vessel models and compared to the Figure 8 design. Computational fluid dynamics (CFD) simulations were also performed on idealized models of the two anastomosis designs to verify the experiments and further study WSS and WSSG profiles of the different designs.
Methods
Ex vivo experiments
Fresh chicken arteries were harvested and connected ex vivo in the Figure 8 and Hybrid configurations using 10-0 nylon sutures (AROSurgial, Newport Beach, CA). Idealized representations of the two configurations are shown in Figure . The Figure 8 anastomosis was constructed by sewing one of the two recipients onto one-half of the donor vessel. The two recipients were then sewn together at the midline using approximately 30% of the circumference of each vessel. In creating the anastomosis, the surgeon attempted to produce the greatest possible cross-sectional area at the point of connection between the two vessels. Finally, the running suture was completed by sewing the second recipient vessel to the other half of the donor vessel.
The Hybrid anastomosis was constructed by making a cut on the inner surface of the two vessels. The length of the incision was approximately 1.5 times the diameter of the vessel. The two vessels were joined forming one half of a side-to-side anastomosis, which was then sutured to the donor artery end-to-end. The Hybrid anastomosis has greater cross-sectional area at the connection site because the entire circumference of each recipient vessel is used in forming the anastomosis.
Three Figure 8 and three Hybrid anastomoses were created by a single surgeon. The time required for completing the anastomosis, donor and recipient vessel diameters, and flow through the anastomosis were recorded for each case. Flow was measured using a modified form of the ‘cut flow’ technique adapted from Charbel et al. (Citation2005). Each anastomosis was connected via the donor vessel to a gravity fed system of normal saline. The fluid from one-liter bag of injectable saline (B. Braun Medical Inc., Bethlehem, PA) flowed through a standard drip set tubing (ALARIS Medical Systems Inc, San Diego, CA) and an 18-gage needle (JELCO, Johnson and Johnson, New Brunswick, NJ). The fluid was maintained at room temperature (20 °C). Elevating the saline reservoir 105–110 cm above the anastomosis produced a steady pressure of 78 mm Hg, which is similar to the intracranial mean arterial pressure in a healthy adult during exercise (Ogoh et al. Citation2005). The volume of fluid that flowed through the anastomosis in 30 s was measured using a calibrated cylinder. Flow rates through the ex vivo models were analyzed using independent sample t-tests.
Numerical simulations
Pressure- and velocity-based inflow boundary conditions were prescribed for CFD simulations of flow through the idealized Figure 8 and Hybrid anastomosis designs to more thoroughly characterize the effects of anastomosis geometry on fluid dynamics. Pressure-based simulations were performed first to validate the experimental model. More physiologically realistic velocity-based simulations were then performed to investigate the impact of geometry on WSS and WSSG.
Model construction
Computational models of idealized Figure 8 and Hybrid anastomoses were constructed using SolidWorks 2010 (SolidWorks Corp., Concordia, MA, USA). The inlet and outlet vessels were modeled as elliptical and cylindrical segments, respectively, based on examples from medical image data. The Figure 8 anastomosis was constructed by connecting the inlet vessel to two halves of a cylinder, while the Hybrid anastomosis was constructed by connecting the inlet vessel to the cylindrical recipient vessels via a funnel-shaped structure that represented the recessed lumen at the anastomosis site (Figure ). Each recipient vessel was constructed with a diameter of 1 mm, and the two vessels were separated by 0.2 mm (representing a vessel thickness of 0.1 mm). The diameter and thickness of the recipient vessels were chosen based on the chicken vessels used in the ex vivo experiments. For each anastomosis design, the computational geometry was constructed under physician supervision starting with the same original unconnected vessels and then proceeding as the actual procedure would take place in the operating room.
Computational fluid dynamics
The inlet of each model was extruded by 20 diameters, which ensured fully developed flow entering the anastomosis site given the Reynolds numbers explored. The fluid volume was discretized into unstructured tetrahedral mesh elements using ANSYS ICEM CFD (ANSYS Inc., Canonsburg, PA, USA). A mesh density function was applied at the anastomosed section to accurately capture the fluid dynamics in that region while minimizing computing time. The Figure 8 anastomosis consisted of approximately 1.9 million mesh elements corresponding to 332,627 nodes and the Hybrid anastomosis consisted of approximately four million mesh elements corresponding to 693,563 nodes. These mesh densities exceeds the threshold that is commonly accepted in our field for quality.
The meshed anastomoses were then imported into ANSYS Fluent 12.1 (ANSYS Fluent Inc., Lebanon, NH, USA) for steady and pulsatile CFD simulations. The models were assumed to be rigid and a no-slip boundary condition was applied at the vessel walls. Fluid properties of saline (Gisladottir et al. Citation2009) for the pressure-based simulations were chosen to match the ex-vivo experiments for validation purposes. More physiologically realistic velocity-based simulations were then conducted with blood modeled as an incompressible and Newtonian fluid with a density of 1060 kg/m3 and viscosity of 3.71 cP (Frauenfelder et al. Citation2007).
Pressure-based simulations
A steady pressure boundary condition of 78 mm Hg was prescribed at the inlet, which matched the inlet pressure imposed during the ex vivo experiments. A range of outlet pressure values was estimated based on calculated pressure drops across the anastomosis site using Poiseuille’s equation (since the pressure boundary conditions for the recipient vessels were unknown before simulation). Eight trials were simulated for each anastomosis with outlet pressures of 75.5, 74.5, 73.5, 72.5, 71.5, 70.5, 69.5, and 68.5 mm Hg.
Velocity-based simulations
Flow rates of 0.2, 0.5, and 0.7 ml/s corresponding to inlet velocities of 0.085, 0.214, and 0.219 m/s were prescribed at the inlet for the steady simulations, and modified MCA waveforms adapted from Reymond et al. were used for pulsatile velocity-based simulations (Stock et al. Citation2000; Reymond et al. Citation2009). Zero-pressure boundary conditions were imposed at the outlets.
Analysis
Tecplot 360 (Tecplot, Inc. Bellevue, WA, USA) was used to analyze the simulation results. For the pressure-based simulations, average flow rates through the recipient vessels were obtained 1 cm downstream to the anastomosis site for each simulation. Based on the slope of the pressure drop vs. computed flow rate, the resistance of each model to blood flow was calculated as:
where R is resistance, ΔP is the pressure drop across the model, and Q is flow rate. Time-averaged WSS (TAWSS) and time-averaged WSSG (TAWSSG) values were computed for both steady and pulsatile velocity-based simulations to understand the effects of anastomosis geometry on shear.
Results
Ex vivo experiments
Time taken to create each anastomosis, donor and recipient vessel diameters, and flow through the ex vivo anastomoses were measured as summarized in Table . The mean suture time averaged 20 min and 25 s for the Figure 8 anastomosis and 28 min and 28 s for the Hybrid anastomosis. The least time taken to create a Hybrid anastomosis (i.e. Trial 3) was comparable to the time taken for the first two Figure 8 anastomoses (i.e. Trials 1 and 2). Diameters of the vessels used were very similar between the two groups, which confirmed that the original donor and recipient vessels used in creating the two different anastomosis designs were roughly equivalent. The average flow rates through the Figure 8 and Hybrid anastomoses were 0.23 and 0.40 ml/s, respectively. Based on independent sample t-tests, the experimentally measured flow rates for the Hybrid anastomosis were significantly greater than those for the Figure 8 anastomosis (p = 0.03).
Table 1. Suture times, donor and single recipient vessel diameters, and flow rates for the two groups of different anastomosis configurations examined with bench-top experiments.
Numerical simulations
Pressure-based simulations
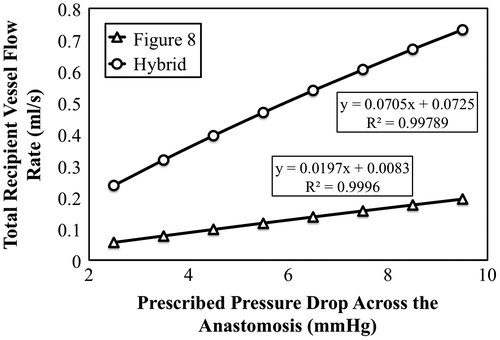
For the steady pressure-based simulation results, average flow rates through the recipient vessels were computed 1 cm downstream of the anastomosis site and plotted against the prescribed pressure drop as shown in Figure . As expected, a linear relationship between flow rate and pressure drop was observed. Based on the inverses of the slopes of lines fitted to the plotted data series, the Figure 8 and Hybrid anastomoses had resistances of 6.77 and 1.89 kPa-s/m, respectively.
Velocity-based simulations
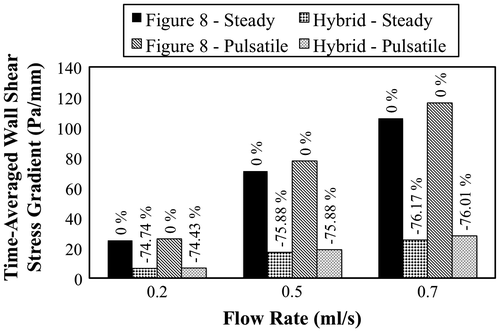
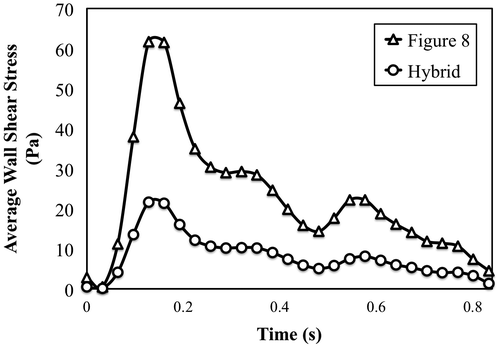
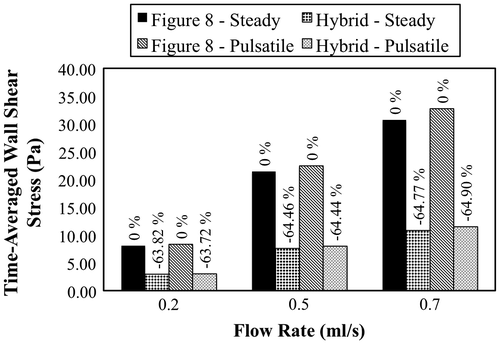
TAWSS and TAWSSG values within the anastomosis region were calculated for all simulations. The results are presented in Figures and . From the plots it is evident that anastomosis geometry plays an important role in determining both WSS and WSSG. Greater than 60% reductions in TAWSS and TAWSSG were observed in the Hybrid anastomosis (as compared to the Figure 8 anastomosis) under both steady and pulsatile flow conditions. Average WSS within the model is also plotted against time for the pulsatile velocity-based simulation in Figure .
Figure 4a. TAWSS computed at the anastomosis site under steady and pulsatile flow conditions at three different flow rates. The percentage change in TAWSS from the Figure 8 to the Hybrid configuration is also reported.
Discussion
Blood vessel geometry is known to play an important role in defining local hemodynamics (Lasheras Citation2007; Sousa et al. Citation2012), and should therefore be considered when designing new anastomosis techniques. In this study, we investigated the effects of a new branching anastomosis design, the Hybrid anastomosis, using experimental, and computational methods. The more common approach to anastomose a donor artery and two recipient vessels, the Figure 8 anastomosis, involves sewing the recipient vessels together at the midline and then attaching them to the donor artery end-to-end. Although this design restores blood flow, the double thickness of the vessel wall at the lumen of the anastomosis may increase WSS and WSSG within the connection. WSSG is known to play a role in modifying endothelial cell response, and high WSSGs may cause intimal hyperplasia (Lei et al. Citation1997; El Zahab et al. Citation2010). The goal of this study was to design a new anastomosis with a recessed central obstruction in order to minimize local WSS and WSSG.
The impact of anastomosis geometry was studied experimentally using ex vivo chicken vessel models, and computationally using CFD. CFD has previously been used as an alternative to in vivo and/or ex vivo flow measurements to quantify the effects of different anastomosis designs on flow (Hughes & How Citation1996; Migliavacca et al. Citation2003; Frauenfelder et al. Citation2007; Kabinejadian et al. Citation2010; Qian et al. Citation2010; Yang et al. Citation2010). The experimental results showed greater flow through the Hybrid anastomosis compared to the Figure 8 anastomosis. Pressure-based CFD simulations validated the experimental results, where resistance curves obtained from the simulation results showed higher flow resistances within the Figure 8 anastomosis compared to the Hybrid anastomosis.
Velocity-based simulations were also performed to study the impact of anastomosis design on vessel biomechanics, in the context of WSS and WSSG, under more physiologically realistic conditions. Simulation results showed that the Hybrid anastomosis reduced TAWSS and TAWSSG by more than 60% under both steady and pulsatile flow conditions. The large reductions in WSS and WSSG indicate that even subtle geometric changes in a branching anastomosis design can play an important role in dictating local hemodynamics, and may be important to promote favorable biomechanical outcomes.
Although this study accomplished its goals by comparing the two anastomosis designs via experiments and simulations, it is not without limitations. First, the ex vivo experiments were performed on chicken vessels perfused with saline at atmospheric pressure rather than in live animal models. Although this scenario would affect overall flow through the vessel, the presented results still provide a telling comparison between the two anastomosis designs on a level playing field. Second, the simulations were conducted using idealized anastomosis designs with rigid walls rather than compliant, case-specific vascular models. Nevertheless, the use of idealized, rigid models facilitated fundamental understanding of the effects that two different design geometries had on flow. Based on previous literature, the global flow and stress profiles generated by compliant wall simulations are similar to those from the rigid simulation case (Zhao et al. Citation2000; Dempere-Marco et al. Citation2006). Accordingly, rather than using our computational resources to accomplish a handful of compliant model simulations, we chose to explore a more extensive range of boundary conditions, which (such as geometry) generally have greater impact on fluid dynamic outcomes (Cebral et al. Citation2011). Using compliant models would of course decrease WSS levels; however, we expect that relative trends across the two different anastomosis designs would not change considerably. Lastly, very simple outflow boundary conditions were used in the CFD simulations. While more advanced (e.g. RCR) boundary conditions could certainly provide greater absolute accuracy in simulation, we once again expect that using such boundary condition models under the assumption of identical outflow conditions for both recipient vessels would not considerably change the relative trends that we observed.
Conclusion
The purpose of this study was to compare the fluid dynamics of two different anastomosis designs. The experimental and computational results both favored the proposed Hybrid anastomosis design over the traditional Figure 8 design. Minimizing the central obstruction within the lumen of the Hybrid anastomosis produced better biomechanical outcomes based on flow resistance, WSS, and WSSG. For example, TAWSS and TAWSSG were both decreased by 60% or more in the Hybrid design under pulsatile flow conditions. Future work will investigate other anastomosis designs toward further advancing the state of the art in vascular surgical capabilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Archie Jr JP, Hyun S, Kleinstreuer C, Longest PW, Truskey GA, Buchanan JR. 2001. Hemodynamic parameters and early intimal thickening in branching blood vessels. Crit Rev Biomed Eng. 29:1–64.
- Charbel FT, Meglio G, Amin-Hanjani S. 2005. Superficial temporal artery-to-middle cerebral artery bypass. Neurosurgery. 56:186–190.10.1227/01.NEU.0000144487.85531.FD
- Cebral JR, Mut F, Weir J, Putman CM. 2011. Association of hemodynamic characteristics and cerebral aneurysm rupture. Am J Neuroradiol. 32:264–270.10.3174/ajnr.A2274
- Cunningham KS, Gotlieb AI. 2005. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 85:9–23.
- Dempere-Marco L, Oubel E, Castro M, Putman C, Frangi A, Cebral J. 2006. CFD analysis incorporating the influence of wall motion: application to intracranial aneurysms. Medical Image Computing and Computer-Assisted Intervention–MICCAI 2006. Berlin: Springer; p. 438–445.
- El Zahab Z, Divo E, Kassab A. 2010. Minimisation of the wall shear stress gradients in bypass grafts anastomoses using meshless CFD and genetic algorithms optimisation. Comput Methods Biomech Biomed Eng. 13:35–47.10.1080/10255840903013555
- Frauenfelder T, Boutsianis E, Schertler T, Husmann L, Leschka S, Poulikakos D, Alkadhi H. 2007. Flow and wall shear stress in end-to-side and side-to-side anastomosis of venous coronary artery bypass grafts. Biomed Eng Online. 6:35–47.10.1186/1475-925X-6-35
- Gisladottir S, Loftsson T, Stefansson E. 2009. Diffusion characteristics of vitreous humour and saline solution follow the Stokes Einstein equation. Graefes Arch Clin Exp Ophthalmol. 247:1677–1684.10.1007/s00417-009-1141-3
- Hanel RA, Spetzler RF. 2008. Surgical treatment of complex intracranial aneurysms. Neurosurgery, 62 SHC1289–SHC1299.
- Hughes PE, How TV. 1996. Effects of geometry and flow division on flow structures in models of the distal end-to-side anastomosis. J Biomech. 29:855–872.10.1016/0021-9290(95)00168-9
- Kabinejadian F, Chua LP, Ghista DN, Sankaranarayanan M, Tan YS. 2010. A novel coronary artery bypass graft design of sequential anastomoses. Ann Biomed Eng. 38:3135–3150.10.1007/s10439-010-0068-5
- Ku DN. 1997. Blood flow in arteries. Annu Rev Fluid Mech. 29:399–434.10.1146/annurev.fluid.29.1.399
- Lasheras JC. 2007. The biomechanics of arterial aneurysms. Annu Rev Fluid Mech. 39:293–319.10.1146/annurev.fluid.39.050905.110128
- Lei M, Archie JP, Kleinstreuer C. 1997. Computational design of a bypass graft that minimizes wall shear stress gradients in the region of the distal anastomosis. J Vasc Surg. 25:637–646.10.1016/S0741-5214(97)70289-1
- Loth F, Fischer PF, Bassiouny HS. 2008. Blood flow in end-to-side anastomoses. Annu Rev Fluid Mech. 40:367–393.10.1146/annurev.fluid.40.111406.102119
- Migliavacca F, Dubini G, Bove EL, de Leval MR. 2003. Computational fluid dynamics simulations in realistic 3-D geometries of the total cavopulmonary anastomosis: the influence of the inferior caval anastomosis. J Biomech Eng. 125:805–813.10.1115/1.1632523
- Ogoh S, Fadel PJ, Zhang R, Selmer C, Jans Ø, Secher NH, Raven PB. 2005. Middle cerebral artery flow velocity and pulse pressure during dynamic exercise in humans. Am J Physiol Heart Circ Physiol. 288:H1526–H1531.
- Ojha M. 1993. Spatial and temporal variations of wall shear stress within an end-to-side arterial anastomosis model. J Biomech. 26:1377–1388.10.1016/0021-9290(93)90089-W
- Okada Y, Shima T, Nishida M, Yamane K, Yamada T, Yamanaka C. 1998. Effectiveness of superficial temporal artery-middle cerebral artery anastomosis in adult moyamoya disease: cerebral hemodynamics and clinical course in ischemic and hemorrhagic varieties. Stroke. 29:625–630.
- Peerless SJ, Mervart JM. 1984. Extracranial-intracranial arterial anastomosis: indications and surgical aspects. Int Anesthesiol Clin. 22:77–88.
- Qian Y, Liu JL, Itatani K, Miyaji K, Umezu M. 2010. Computational hemodynamic analysis in congenital heart disease: simulation of the Norwood procedure. Ann Biomed Eng. 38:2302–2313.10.1007/s10439-010-9978-5
- Reymond P, Merenda F, Perren F, Rüfenacht D, Stergiopulos N. 2009. Validation of a one-dimensional model of the systemic arterial tree. Am J Physiol Heart Circ Physiol. 297:H208–H222.10.1152/ajpheart.00037.2009
- Sousa LC, Castro CF, António CC, Chaves R. 2012. Blood flow simulation and vascular reconstruction. J Biomech. 45:2549–2555.10.1016/j.jbiomech.2012.07.033
- Stock KW, Wetzel SG, Lyrer PA, Radü EW. 2000. Quantification of blood flow in the middle cerebral artery with phase-contrast MR imaging. Eur Radiol. 10:1795–1800.10.1007/s003300000378
- Tzakis AG, Reyes J, Nour B, Marino IR, Todo S, Starzl TE. 1993. Temporary end to side portacaval shunt in orthotopic hepatic transplantation in humans. Surgery, Gynecol Obstet. 176:180–182.
- Yang N, Deutsch S, Paterson EG, Manning KB. 2010. Hemodynamics of an end-to-side anastomotic graft for a pulsatile pediatric ventricular assist device. J Biomech Eng. 132:031009.10.1115/1.4000872
- Zhao SZ, Xu XY, Hughes AD, Thom SA, Stanton AV, Ariff B, Long Q. 2000. Blood flow and vessel mechanics in a physiologically realistic model of a human carotid arterial bifurcation. J Biomech. 33:975–984.10.1016/S0021-9290(00)00043-9