Abstract
Objective: To study the effect of two different microemulsions containing Beackea frutescence supplements composed of nerolidool, selenium and vitamin E on absorption effect related to skin health and skin aging.
Materials and methods: A total of 39 volunteers with normal and healthy skin were divided into three groups (n = 13) and supplemented for a period of 12 weeks. Group 1 received a mixture of lutein (3 mg/day), lycopene (3 mg/day), α-tocopherol (10 mg/day), selenium (75 μg/day) and β-nerolidool (4.8 mg/day) and Group 2 was supplemented with a mixture of β-nerolidool (4.8 mg/day), lycopene (6 mg/day), selenium (75 μg/day) and α-tocopherol (10 mg/day). Group 3 was the placebo control. Wrinkling, smoothness, scaling and roughness of the skin were determined by Surface Evaluation of Living Skin (Visioscan).
Results: Upon supplementation, serum levels of selected nerolidool increased in both groups. Skin thickness and density were determined by ultrasound measurements. A significant increase for both parameters was determined in the serum groups. Roughness and scaling were improved by the supplementation with antioxidant micronutrients. In the placebo group, no changes were found for any of the parameters.
Conclusion: Beackea frutescence microemulsion supplements have shown significant change in the texture of human skin as well as scaling, wrinkling, smoothness and roughness were improved by the supplementation.
Introduction
Beackea frutescence (cucur atap) is a member of Myrtacae family, and is well known as a aromatic and medicinal herb.[Citation1] This species has an attractive weeping shrub with yellow flower. It can grow upto 3 meters long and it can be easily found on sandy coast of the east coast of Peninsular Malaysia. This plant contains about 2.0% of essential oils with a sweet odor, which is viable for essential oil production and is suitable as a natural spurce for fragrance.[Citation2] The major components of nonvolatile constituent of this plant are nerolidool, phloroglucinols flavanones, chromones and chromanones, chromone and C-glycosides.[Citation3] The supplements of Beackea frutescence essential emulsions are widely used as skin protectants and supplementation with nerolidool has been shown to protect against UV-induced erythema. However, little is known about the other effects of nerolidool on skin health. The maintenance of skin health considerably depends on the amounts of antioxidant micronutrients such as vitamins and nerolidool that are present in the skin.[Citation4] It is also convincingly proved that antioxidants can recover the skin damaged due to sunlight.[Citation2,Citation3,Citation5–7] It has been shown that vitamin C and nerolidool, prevent sunburn-associated erythema following UV exposure. Reactive oxygen species in the skin is generated by UV radiation, leading to damaging reactions, which have been associated with premature skin ageing, photosensitivity or photocarcinogenesis.[Citation8,Citation9] Thus, initiated the mechanism of action underlying skin protective effects of antioxidants by scavenging reactive oxygen species.[Citation10,Citation11] However, it has been proved that several antioxidants exhibit biological properties indirectly related to antioxidant activities. They may trigger cell cycle progression, influence cellular signaling pathways, cell growth and repair systems.[Citation12,Citation13] Only a few information available on the effects of nerolidool, vitamins and trace elements on skin texture and structure is closely related to skin ageing.[Citation14–16] Pre-mature aging of the skin results in loss of elasticity and increased wrinkling. This study will explore whether the absorption supplementation of Beackea frustescence emulsions with micronutrients modulates skin structure contributing to the resistance and improving general parameters indicative of skin health.
Methodology
Study design
This study was carried out as a placebo-controlled supplementation, monocentric study on the effects of antioxidant micronutrients on parameters of skin health and skin aging. In all, 39 volunteers (10 males and 29 females, average age: 25 years) with healthy normal skin were included in the study. They were divided into three groups of 13 test subjects each (two serum and one placebo group). Dosing in the different treatment groups and their composition of the antioxidant supplements are summarized in . All volunteers were adviced not to change their dietary habits during the study. Every test subject had to submit a written declaration of consent for their participation in the study. Skin parameters were determined before starting the supplementation (week 0) and at week 6 and 12; blood samples for antioxidant analyses were taken at the same time points and the supplementation period lasted for 12 weeks. All test subjects received detailed information listing every single parameter relevant to the study.
Table 1. Treatment allocation, dose/day in the three different treatment groups.
Inclusion criteria
The strict inclusion criteria were as follows: nonsmoking, age 18–65 years, not pregnant or lactating, normal nutritional habits, no intake of vitamin supplements, no history of photosensitizing disorders, no history of mal-absorption diseases, liver diseases or diseases of lipid metabolism during the study. Screening procedures were carried out to check the general health of the participants and their medical history.
Parameters of skin structure
Skin density and thickness were determined by means of ultrasound (B-scan), and skin surface was evaluated by image analysis (Surface Evaluation of Living Skin, SELS; Visioscan) at week 0, 6 and 12.
B-scan
An ultrasound device with a frequency of 20 MHz (Derma Scan C, with 2-D configuration, Cortex Technology, Denmark) was used for noninvasive differentiation of individual tissue structures.[Citation10] Skin thickness (dermis and epidermis) is given in millimeters. Two hundred and fifty-six randomly chosen colors are assigned to the different echo amplitudes, which helps to determine even the slight differences in the reflection behavior. Pixel density was used to determine the skin density.
Surface evaluation of living skin (SELS)
The skin surface is described by four different parameters: roughness, scaling, smoothness and wrinkling.[Citation11] Skin surface analysis according to the SELS method is based on the evaluation of an image of living skin taken under certain illumination; the picture is electronically processed for quantitative analyses. The measuring device consists of a measuring head containing two contra-rotating metal halogen lamps evenly illuminating a 15–17 mm measuring field on the skin. A CCD camera, located in the measuring head, records a picture of the skin, which is then transferred as graded gray values into a bitmap file (software: Skin-Visiometer, Courage & Khazaka, Cologne). The method allows to differentiate between 256 possible gray values. By means of the additional software (SELS), the skin-specific parameters are then calculated. The spectrum of the lamps and their intensity as well as their location allows to analyze the skin surface without interfering reflections from deeper layers.
Antioxidants in serum
Blood samples were taken after overnight fasting at week 0, 6 and 12; serum was prepared by centrifugation and stored at –80° C until analysis. Lycopene, β-norolidool, α-nerolidool, lutein, zeaxanthin and cryptoxanthin were determined by HPLC (Bennefotte, PA) as described by Stahl et al. [Citation3] Additionally, phytoene, phytofluene, α phloroglucinols and γ- phloroglucinols were determined. For phytoene and phytofluene analyses, 500 μl of serum were extracted with hexane/dichloromethane (5/1, v/v, stabilized with 0.1% 2,6-di-tert-butyl-p-cresol) after protein precipitation with ethanol. The organic solvent was evaporated to dryness under a stream of nitrogen, and the residue was dissolved in 100 μl dichloromethane and 100 μl acetonitrile. The HPLC system consisted of a Merck-Hitachi L-7100 pump connected with a Merck-Hitachi UV/Vis detector and an integrator for data registration. HPLC was performed isocratically with an eluent consisting of acetonitrile/methanol (85/15, v/v) and a reversed-phase column (pKb-100, 250 × 4.6 mm, Supelco, Bellefonte, PA) protected by a guard column (4.6 × 4.6 mm) with the same stationary phase. The flow rate was set to 1 ml/min. A second UV/Vis detector was connected in series and set at 325 and 292 nm for quantitation of retinol, α-phloroglucinols and γ-phloroglucinols.[Citation12,Citation13] The concentrations were calculated from external calibration curves generated with original standard compounds. In a separate run under the same conditions, phytofluene was detected at a wavelength of 348 nm and phytoene at 278 nm.
Statistical method
For all time points, descriptive statistics and all parameters were calculated; pre-post differences were calculated and analyzed descriptively. Within the three treatment groups, each combination of two time points was compared using the Wilcoxon signed rank test. For the pre–post differences, each combination of two treatment groups was compared using the Wilcoxon rank sum test. Percentual changes of all measured parameters were determined and the p values were calculated at all measuring points.
Results
Ultrasound measurements (B-scan)
In both treatment groups, a statistically significant increase (p < 0.05) in skin thickness and skin density was observed after 6 and 12 weeks, compared to week 0 ( and ). No statistically significant changes were observed in the placebo group. Skin density increased in groups 1 and 2 by about 7%, skin thickness by about 15%. No differences were found between treatment groups. A typical ultrasound B-scan is presented in . It shows the density and thickness of the dermis before and after 12-week treatment of a volunteer from group 1.
Figure 1. Increase in skin thickness (dermis and epidermis; (a) and density (b) during supplementation. Skin density and thickness were measured by means of ultrasound (B-scan).
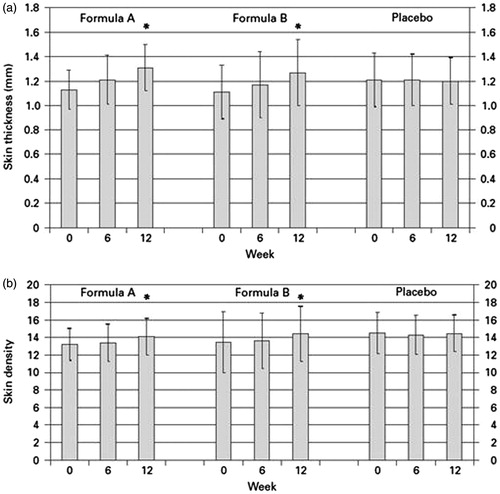
Figure 2. Skin density before (a) and after 12 weeks of treatment with formula A (b). A typical ultrasound B-scan shows the density and thickness of the dermis before and after 12 weeks of treatment; obtained from a volunteer of group 1.
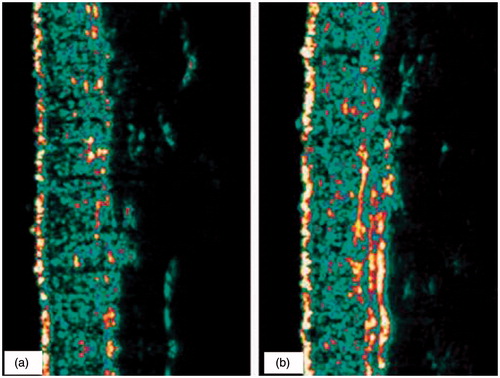
Table 2. Results and statistical evaluation of the ultrasound measurements, skin density and skin thickness.
Surface evaluation of living skin
SELS provides parameters related to the structure of the skin surface including scaling, roughness, smoothness and wrinkling (, ). A typical SELS picture is presented in . It shows the surface of the skin of a volunteer from group 2 (formula B) before and after 12 weeks of treatment as well as a 3-D image.
Figure 3. Skin surface evaluation. Surface of the skin of a volunteer from group 2 (formula B) before (a) and after 12 weeks of treatment (b); CCD picture and 3-D computerized image. Skin surface was evaluated by image analysis (SELS).
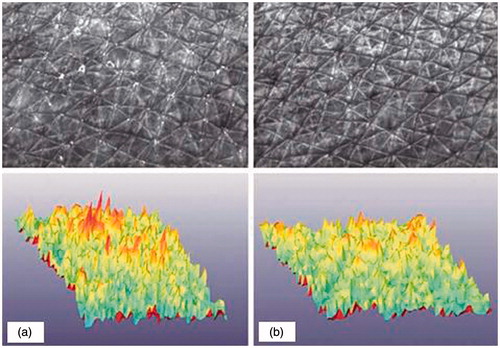
Figure 4. Decrease in roughness (a) and scaling (b) during supplementation. Skin surface was evaluated by image analysis (SELS).
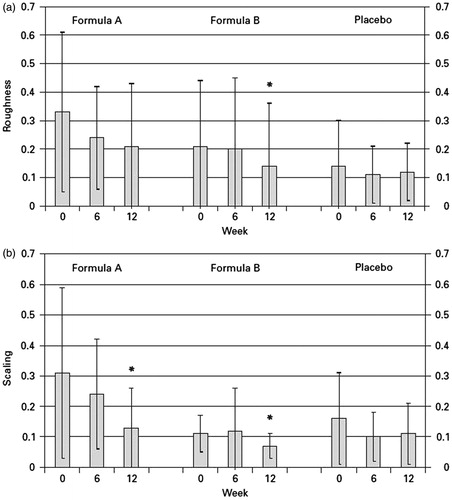
Table 3. Results and statistical evaluation of the skin surface evaluation.
Following surface evaluation of living skin, scaling was affected by supplementation with antioxidants. In both the treatment groups, a statistically significant decrease was observed after 12 weeks (p < 0.05). For the parameter roughness, only in the group 2, a statistically significant decrease was found (p < 0.05). Changes in scaling and roughness determined in the placebo group were statistically not significant. Other SELS parameters, i.e. smoothness and wrinkling, were not affected by treatment and did not change in any of the groups (data not shown).
Antioxidants in serum
The nerolidool, lycopene, β-nerolidool, lutein, zeaxanthin, β-nerolidool, cryptoxanthin, phytoene and phytofluene as well as α-phloroglucinols, γ-phloroglucinols and retinol were analyzed in blood samples from all groups (). The baseline levels of the micronutrients analyzed were comparable in all groups and are within the range of variance reported in the literature.[Citation13,Citation14] In group 1, lycopene and β-nerolidool levels increased to yield final concentrations (week 12) of 0.76 and 1.62 nmol/ml, respectively. Although the dose of lycopene was low in this supplement, relative increases were comparable to those observed in group 2. Phytoene and phytofluene serum concentrations were elevated after treatment; the values are comparable between both treatment groups. The supplement used in group 1 contained 1.5 mg lutein/capsule. Lutein levels increased from baseline 0.23 to 0.63 nmol/ml at the end of the study. Additionally, zeaxanthin increased. No changes in serum levels were found for cryptoxanthin, α-carotene and retinol (data not shown). None of these compounds was present in the supplement. α-Phloroglucinols levels were slightly elevated after treatment; γ-phloroglucinols remained unchanged. In group 2, lycopene and β-nerolidool levels increased to yield final concentrations (week 12) of 0.88 and 1.33 nmol/ml, respectively. Both compounds were present in the supplement. Increases in phytoene (level at week 12: 0.15 nmol/ml) and phytofluene (level at week 12: 1.29 nmol/ml) were also measured. Both nerolidool are precursors of lycopene and β-nerolidool and are present in nerolidool supplements that contain oleoresins. The increased levels are within the range from the previous studies.[Citation4] It should be noted that the bioavailability of nerolidool depends on several endogenous and exogenous factors leading to a wide range of variation in the individual uptake of single compound. Serum responses vary between nerolidool and are not always linear with the dose.
Table 4. Antioxidants in serum (nmol) determined by HPLC analysis and statistical evaluation.
Statistical evaluation of the serum concentration of antioxidants
For the treatment with formula A/group 1, statistically significant increases (p < 0.05) were evaluated for all antioxidants in serum after 12 weeks compared to week 0. For the treatment with formula B/group 2, statistically significant increases (p < 0.05) for the antioxidants α-nerolidool, lycopene total, all-trans lycopene, phytoene and phytofluene were observed after 12 weeks compared to week 0. For lutein and α-phloroglucinols, no statistically significant changes were observed during the study. In the placebo group, no statistically significant changes were observed.
Discussion
Endogenous and environmental factors have an effect on skin structure and function with either beneficial or adverse effects on skin health. Variety of skin care products is available to optimize the skin conditions; however, dietary constituents may also influence skin parameters including color, moisture, texture and other physiological properties. Skin requires an optimal supply with nutritive compounds as any other tissue, including macronutrients such as amino acids, lipids, or carbohydrates and micronutrients including essential minerals and vitamins.[Citation13,Citation14] Other nutritive compounds and lipids are applied topically in order to improve skin conditions. A few information is known about the effects of endogenous supply of selected dietary components on skin tissue. Vitamins C and E as well as nerolidool, like β-nerolidool and lycopene are used as nutritional supplements and photoprotective effects have been assigned to these compounds. Several intervention studies have shown their efficacy in preventing UV-induced erythema in.[Citation16,Citation17] In recent study, we demonstrated that a supplement mixture consisting of nerolidool, vitamin E and selenium increases skin density and thickness when ingested over a period of 12 weeks. Also, skin surface parameters including roughness and scaling are improved upon supplementation. The biochemical mechanisms underlying the effects of such a mixture of micronutrients are poorly understood. All components of the supplement are either direct or indirect antioxidants. Topical and/or systemic application of antioxidants has suggested that under stress conditions, these contribute to the maintenance of healthy skin barrier.[Citation4] Interactions between structurally different compounds with variable antioxidant activity may provide additional protection against increased oxidative stress. The antioxidant defense system of the organism is a complex network and comprises several antioxidants. For example, there is evidence from in vitro studies, that β-nerolidool regenerates phloroglucinols from the phloroglucinols radical. Scavenging reactive oxygen species may be one part of their biological activity.
It has been discovered that nerolidool and vitamin E additionally modulate signaling pathways independent of their antioxidant properties. Such modulating effects have been reported in cultured human cells [Citation1] and should be considered as further possible mechanisms underlying the effects of micronutrients on skin properties and skin health. Skin aging is a continuous process and results from intrinsic and extrinsic factors. A number of extrinsic, or external, factors often act together to prematurely age our skin. Most premature aging is caused by sun exposure and affects parameters of skin structure and surface. It is not known yet if the process of premature aging of the skin can be modulated by dietary antioxidants. However, several studies, including the present one, show that dietary antioxidants improve skin structure and provide photoprotective effects when administered as food supplements.[Citation14,Citation15] This study embraces the efficacy of the ingested micronutrients and their influence on the structural changes. All ultrasound and skin surface measurements were carried out on the forearm of the volunteers to avoid mimic influences during the test procedure. The accumulation of the tested formulations in skin and serum could be demonstrated as well as the efficacy of the supplementation in terms of skin structure, like skin surface and skin density.
Acknowledgements
The authors would like to thank Mr Mohd Nasrul Yahya of Biotechnology Laboratory, Technology Park Malaysia Bukit Jalil for carrying out the supplementation test and statistical evaluation of the data and also to all the volunteers who made this investigation possible.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
Ministry of Higher Education Malaysia (MOHE) granted this research under Fundamental Research Grant Scheme [FRGS59262/01/2014].
References
- Habsah M, Muhammad Amin M, Rosmeira Zulhelmi MA, et al. Comparison of hydrodistillation methods for extraction of essential oils from Beackea frutescences and evaluation for the antioxidant activity. J Sustainabil Sci Manage. 2008;3:66–75.
- Boelsma E, Hendriks HF, Roza L. Nutritional skin care: health effects of micronutrients and fatty acids. Am J Clin Nutr. 2001;73:853–864.
- Aust O, Stahl W, Sies H, et al. Supplementation with tomato-based products increase lycopene, phytofl uene and phytoene levels in human serum and protects against UV-induce erythema. Int J Vit Nutr Res. 2005;75:54–60.
- Sies H, Stahl W. Nutritional protection against skin damage from sunlight. Annu Rev Nutr. 2004;24:173–200.
- Ng DSH, Laili CR, Hamdan S, et al. Essential oil of kaffir lime in system of H2O/tween 80/hexane. J Ultra Sci Phys Sci. 2009;21:137–144.
- Ng DSH, Laili CR, Hamdan S, et al. Emulsion systems with Citrus hystrix stabilized by mixed non-ionic surfactants. J Ultra Sci Phys Sci. 2009;21:425–434.
- Hongratanaworakit T, Buchbauer G. Chemical compositions and stimulating effects of Citrus hystrix oil on humans. Flavour Frag J. 2007;22:443–449.
- Bakkali F, Averbeck S, Averbeck D, et al. Biological effects of essential oils-a review. Food Chem Toxicol. 2008;46:446–475.
- Rozaini MZH, Brimblecombe P. The solubility of sodium dicarboxylate in the atmospheric aerosols. J Chem Thermodyn. 2009;41:980–983.
- Lertsatitthanakorn P, Taweechaisupapong S, Aromdee C, et al. In vitro bioactivities of essential oils used for acne control. Int J Aromather. 2006;6:43–49.
- Nanasombat S, Lohasupthawee P. Antibacterial activity of crude ethanolic extracts and essential oils of spices against salmonellae and other enterobacteria. J KMITL Sci Tech. 2005;5:527–538.
- Kongtun S, Suracherdkaiti W. Herbal antibacterial liquid soap development against bacterial causing skin diseases. In: Mendez-Vilas A, editor. Current research topics in applied microbiology and microbial biotechnology. 1st ed. Singapore: World Scientific Publishing; 2009. p. 497–500.
- Breuer MM. Cosmetic emulsions. In: Becher P, editor. Encyclopedia of emulsion technology, Vol. 2. New York: Marcel Dekker; 1983. p. 385–424.
- Holmberg K, Jönsson B, Kronberg B, et al. Surfactants and polymers in aqueous solution. 2nd ed. England: John Wiley & Sons; 2003. p. 156–160.
- Fox C. Cosmetic Emulsions. In: Lissant KJ editor. Emulsions and emulsion technology part 2. surfactant science series, Vol. 6. New York: Marcel Dekker; 1974. p. 701–933.
- Ekwall P. Compositions, properties and structures of liquid crystalline phases in systems of amphiphilic compounds. In: Brown GH, editor. Advances in liquid crystals, Vol 1. New York: Academic Press; 1975. p. 1–142.
- Orafidiya LO, Adesina SK, Igbeneghu OA, et al. The effect of honey and surfactant type on the antibacterial properties of the leaf essential oil on Ocimum gratissimum Linn. against common wound infecting organisms. Int J Aromather. 2006;16:57–60.
