Abstract
Purpose: Some patients experience adverse reactions to poly(methyl methacrylate)-based (PMMA) dentures. Polyamide (PA) as an alternative to PMMA has, however, not been well documented with regard to water sorption and water solubility. The aim of this in vitro study was to measure water sorption and water solubility of two PA materials compared with PMMA, and to evaluate the major components released from the PA materials and the effect on hardness of the materials.
Methods: Ten discs (40.0 mm diameter, 2.0 mm thick) of each material (PA: Valplast and Breflex; PMMA: SR Ivocap HIP) were prepared according to manufacturers’ recommendations. The specimens were tested for water sorption and water solubility, according to a modification of ISO 20795-1:2008. Released substances were analysed by gas chromatography/mass spectrometry (GC/MS).
Results: There were statistically significant differences among the materials regarding water sorption, water solubility and time to water saturation. Breflex had the highest water sorption (30.4 μg/mm3), followed by PMMA-material (25.8 μg/mm3) and Valplast (13.6 μg/mm3). Both PA materials had statistically significant lower water solubility than the PMMA. Both PA had a net increase in weight. Analysis by GC/MS identified release of the compound 12-aminododecanolactam from the material Valplast. No release was found from the Breflex material.
Conclusions: The PA denture materials show differences in water sorption and solubility, but within the limits of the standard requirements. The PA showed a net increase in weight after long-term water sorption. The clinical implications of the findings are not elucidated.
Keywords:
1. Introduction
There are several challenges with the use of removable partial dentures. Most denture materials are hard and can cause mechanical traumas and discomfort. Many patients experience problems with loose and poor fitting dentures and would like to have a denture that can be more securely fitted to the gums and remaining teeth. One study reported that only 64% still used their removable partial dentures regularly one year after insertion [Citation1] while other studies report higher patient satisfaction [Citation2,Citation3]. The most commonly used materials for removable partial dentures are combinations of metal alloys and poly(methyl methacrylate) (PMMA) to support the denture teeth. Fabrication is complicated, time-consuming and costly. Fractures and wear of both metal and PMMA occurs frequently and repairs are difficult. Furthermore, denture base materials have a potential to cause adverse reactions due to release of monomers and biofilm-related infections [Citation4–6]. Since the dentures are in direct contact with large areas of the oral mucosa there is potential for fungi infections and allergic reactions [Citation7–10]. Finally, the aesthetics of conventional removable partial dentures is not optimal, with metal clasps visible on the buccal side of the teeth.
Several materials have been suggested as alternatives to PMMA. Polyamide (PA), or nylon, was introduced as a flexible alternative in the 1950s [Citation11]. Nylon was initially the trade name for a synthetic polymer from the company DuPont. Nylon is now a generic term for a group of polymers that are made of aliphatic chains linked by amide bonds (–CO–NH–) with the chemical term PA [Citation12]. The two terms are used interchangeably. Depending on the chemical structure of the PA, different designations may be used for the material, such as nylon-6,6; nylon-6-10; or nylon-12, referring to the building blocks used in the production of the material.
The PA denture base material was intended to solve the problems of allergies and mechanical trauma of the hard PMMA dentures. The early PA rapidly lost color, shape and stability due to continuous water uptake [Citation11,Citation13]. Polyamide dentures were therefore not a clinical success. Lately, PA has been re-introduced as an alternative to PMMA. The manufacturers advocate PA as a flexible, allergy free, aesthetic alternative for removable partial dentures. The modern PA have different chemical composition than the one used in the 1950s but the exact chemical structure is not always disclosed by the manufacturers [Citation14]. Only a handful of studies have been performed with the modern materials and little evidence is available [Citation7,Citation12,Citation15–19]. Two recent reviews present the existing knowledge regarding material properties and clinical application [Citation20,Citation21].
It is uncertain whether the previous problems of water sorption and lack of stability have been solved for the modern PA. The aim of this study was to compare the water sorption and water solubility of two different commercially available PA materials and a conventional PMMA, and to evaluate the major components released from the PA materials. In addition, the effect of water sorption on the hardness of the materials was investigated.
2. Materials and methods
Test specimens of a heat-polymerized PMMA (SR Ivocap High Impact Polymer [HIP]) and two injection molded PA resins (Breflex and Valplast) were investigated (). A modification of a standardized method for measuring water sorption and solubility [Citation22] was used to achieve values of sorption and solubility for a prolonged time. Ten discs of each material were made by dental technicians according to the manufacturers’ instructions. The specimens were wet-grinded and polished to the size of 2.0 ± 0.2 mm thick and 40 ± 0.2 mm in diameter using FEPA P#220 silicon carbide paper (Struers, Ballerup, Denmark). The volume (V, in mm3) of each specimen was calculated using the mean of 3 diameter measurements and the mean of 5 thickness measurements (one in the center and four equally spaced around the circumference). The specimens were placed in racks inside desiccators (DURAN Group GmbH, Wertheim, Germany) containing freshly dried silica gel (Merck KGaA, Darmstadt, Germany) and stored at 37 ± 1 °C for 23 ± 1 h and weighed on an analytical balance, accurate to 0.0002 g (Sartorius AG, Goettingen, Germany). The drying and weighing cycle was repeated until constant mass, m1, called ‘conditioned mass’, was reached. The specimens were then immersed in water at 37 ± 1 °C. The specimens were removed from water after five days, wiped gently until free from visible moisture and weighed 60 s after removal. The specimens were re-immersed in water and the measurement procedure repeated every 4–5 days until constant mass was reached, m2, called ‘water saturation’. The specimens were then removed from the water and replaced in the desiccator. The desiccation procedure described above was repeated until constant mass was reached, m3, called ‘reconditioned mass’. The water sorption, Wsp, and water solubility, Wsl, were expressed in μg/mm3 using the following equations:
Table 1. Materials used in this study.
where m1 is the constant mass dry specimens, m2 is the constant mass wet specimens and m3 is the constant mass reconditioned specimens, all values in μg, V is the volume of specimens in mm3.
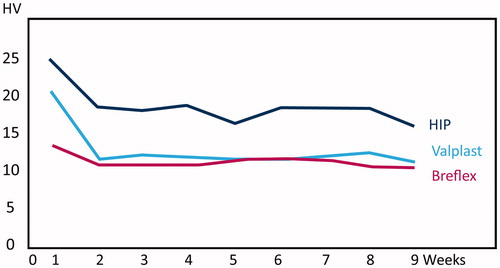
Hardness was measured by the Vickers indentation technique on dry specimens before immersion in water and then subsequently once a week for 9 weeks. The mean of three measurements on each specimen was used in the analyses.
Chemical analysis was performed on unprocessed materials (Breflex and Valplast) that were dissolved separately in different solvents in order to evaluate which components that may leach out of the materials. Analysis was performed by gas chromatography/mass spectrometry (GC/MS). GC system: 5975 Series, mass selective detector (MSD): model 63170A, column: HP 5MS (all from Agilent Technologies, Santa Clara, CA). A temperature program was used for the detection of substances in the materials. Identification was done using a database (NIST MS Search 2.0, NIST, Gaithersburg, MD) combined with retentions times and reference material (12-Aminododecanolactam, 12-ADL, CAS-number: 947-04-6, purity 98%, Sigma-Aldrich, St. Louis, MO).
For quantification of released substances in the processed materials three additional specimens of each material were made by dental technicians, according to the manufacturers’ instructions. The size of the specimens was 2.0 ± 0.2 mm thick and 10.0 ± 2.0 mm in diameter. Each specimen was cut into smaller parts. From each of the specimens, the pieces were accurately weighed into two vials and 3 ml 75% ethanol solution was added. Elution was performed for 72 ± 1 h at a temperature of 37 ± 1 °C. Analyses was done by GC/MS using a selected ion monitoring (SIM) method and isothermal conditions (200 °C, 7 min). Each sample solution was analyzed twice. A calibration curve was prepared for 12-ADL using linear regression (R2=0.997) and the quantifier ion 197 m/z (qualifier ions 41, 55, 100 m/z).
Statistical analyses were performed using a statistical software package (IBM SPSS, 21, Chicago, IL). Comparison among groups was performed with one-way ANOVA, and between groups with Tukey’s post hoc tests. The level of significance was set to .05.
3. Results
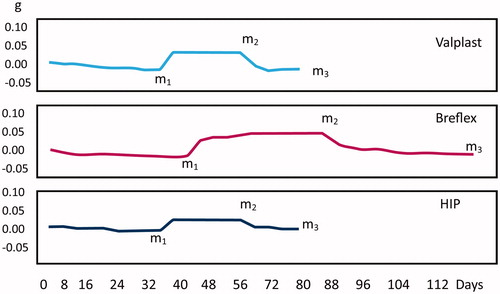
There were statistically significant differences among the materials regarding water sorption, water solubility and time to water saturation (m2) (p < .05, and ). For the water solubility, both Breflex and Valplast had a net increase in weight, giving rise to negative solubility values. All of the tested denture base materials fulfilled the requirements of ISO 20795-1 regarding water sorption (<32 μg/mm3) and solubility (<1.6 μg/mm3). The time to water saturation was 45 days for Breflex, 35 for Valplast and 32 days for HIP. Time to reconditioning was 38 days for Breflex, 16 for Valplast and 15 days for HIP. There was a statistically significant difference in hardness among the materials (p < .05) and all materials revealed a statistically significant reduction in hardness over time ().
Figure 1. Mean values of water sorption and solubility of the tested materials in μg/mm3. Whiskers represent the standard deviation. There were statistically significant differences among all groups for both sorption and solubility (p < .05).
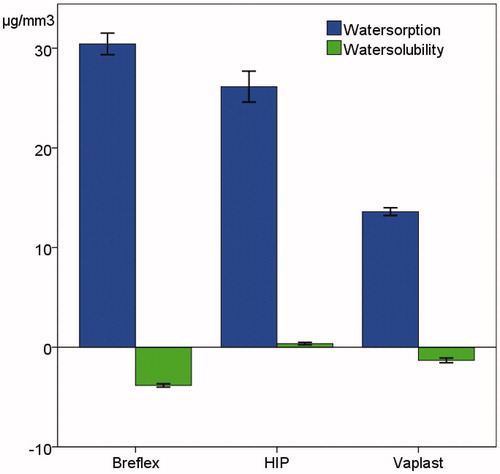
Figure 2. The mean difference in weight over time of specimen discs measured in g. The specimens were inserted in water after reaching a constant mass at m1 and removed from water at m2 (constant mass wet specimens). The reconditioned mass was determined as m3.
Analysis by GC/MS identified the compound 12-aminododecanolactam in the material Valplast, The release of 12-aminododecanolactam from Valplast was quantified to 0.17 ± 0.01 ‰ (per mille) in ethanol solutions after 72 h at 37 °C. No monomer could be identified in the pellets from the Breflex material, however signals from benzophenone were found. No substances could be detected to release from the Breflex specimens. Release from PMMA was not evaluated.
4. Discussion
The findings show that both the Breflex and Valplast PA meet requirements regarding water sorption and solubility given in the standard ISO 20795-1, measured according to a modified method. However, both materials retain water, shown by the negative solubility values. The reduction in hardness indicates that detrimental effect on the mechanical properties occurs due to water uptake. Water may break chemical bonds within the material and reduce fracture strength and alter both size, strength and flexibility [Citation23]. The PA materials in this study are thermoplastic and pre-polymerized by the manufacturer, and are formed to the desired shape using heat. Polyamide is a generic term indicating that the macromolecule is linked by amide groups. However, the generic term does not indicate if there are chemical differences between two materials, such as polyamide-6 (PA-6) or polyamide-12 (PA-12) indicating the number of carbon-atoms between the amide bonds in the polymer. This will give different properties of the two materials. The results indicate that there may be a difference among the tested PA materials. 12-ADL was released from the Valplast material. This compound is a common building block for the production of nylon of the type called PA-12. Limited information regarding type of PA in the information from the manufacturer makes the interpretation of these findings difficult.
In processed Breflex, no substances were detected that could be interpreted as being residual ‘monomers’ or degradation products from the material. However, signals from benzophenone were found, which could be a possible photo-initiator and UV-stabilizer in the material. Also signals from an unidentified compound, most likely a dye, possibly an acridine-type, were found. These substances are additives in the material and their release was not quantified. In this study, we did not evaluate the released product from the PMMA-material, as this has been done for similar materials [Citation24–26]. In a previous study including both a thermoplastic and a powder and liquid-based (P&L) PMMA material, it was found that the release into water of methyl methacrylate monomer was 100 times larger for the P&L material than for the thermoplastic material [Citation27].
The results on water sorption and solubility ( and ) revealed that the PA continue to absorb water for up to 8 weeks. The Breflex-material had a statistically significant longer time until stable reconditioned mass compared to the other materials tested which indicates that the clinical problems experienced previously may not have been fully solved [Citation14]. The standard for testing of denture materials [Citation22] uses a shorter time for the evaluation of water sorption. This will not register the continuous water sorption for some materials as seen in the present results. Takabayashi found a difference regarding water sorption between three different PA materials compared to a PMMA [Citation17]. One of the PA had higher water sorption than the PMMA, whereas the two others had lower water sorption than the PMMA concurring the findings in the present study.
The amide groups in PA are polar and may form intra- and inter-chain hydrogen bonds. Hydrogen bonds may also be formed between PA and water molecules, and nylon material will absorb some water. The polarity of nylon material will vary with the chain length between the amide groups, the shorter the chain, the more hydrophilic is the material. Thus, a PA-6 material will be more hydrophilic than a PA-12 material, and higher water sorption may be expected. Information from the manufacturer of Breflex announces the material to be PA-12 and the detection of 12-ADL in Valplast indicates that this material is a PA-12 as well. However, the results from the water sorption study indicate that Breflex is more hydrophilic than Valplast. Differences in the manufacturing techniques or different additives in the two materials may cause the observed differences. Whether this will affect the materials’ mechanical properties over time is not evident from the present study, but it is reasonable to expect degradation in flexural strength over time with due to the water sorption.
In this study, PMMA had the highest hardness value, both before and after water sorption. Still, the three materials all showed important decrease in hardness upon water immersion, specifically within the first week (). There seems to be no direct correlation between the amount of water sorption and the reduction in hardness; Breflex having the highest water sorption conversely had the smallest reduction in hardness. Very little scientific research has been performed on the modern PA materials. The mechanic properties are studied in some in vitro studies [Citation12,Citation15–18]. The results reveal poorer mechanical properties than PMMA and a tendency to discolor when immersed in strongly colored liquids although there are lager variations among the materials [Citation16]. It is, however, complicated to make direct comparison between the PA materials and PMMA. The PMMA is hard and brittle while the PA are flexible and the materials are used differently even though they are used in the same clinical situations. The PMMA engages undercuts for retention via metallic clasps, while the PA dentures are made of the same material throughout. It is thus uncertain whether the standard test methods for denture base materials are applicable or not.
5. Conclusions
The PA denture materials showed differences in water sorption and solubility, but within the limits of the standard requirements. One of the tested PA had continuous water sorption up to 8 weeks. Whether this will represent a clinical problem or not is not yet evident. One of the studied PA released small amounts of a monomeric structure. The hardness of both PA and the PMMA-material was reduced upon water immersion.
Acknowledgements
Hanne Wellendorf is acknowledged for her work with the analyses of substances and release. Odd Johan Lundberg is acknowledged for assistance with technical procedures.
Disclosure statement
None of the authors or their institutions have any conflicts of interest with any of the products mentioned.
Additional information
Funding
References
- Akeel R. Usage of removable partial dentures in Saudi male patients after 1 year telephone interview. Saudi Dent J. 2010;22:125–128.
- Rehmann P, Orbach K, Ferger P, et al. Treatment outcomes with removable partial dentures: a retrospective analysis. Int J Prosthodont. 2013;26:147–150.
- Zlataric DK, Celebic A. Treatment outcomes with removable partial dentures: a comparison between patient and prosthodontist assessments. Int J Prosthodont. 2001;14:423–426.
- Weaver RE, Goebel WM. Reactions to acrylic resin dental prostheses. J Prosthet Dent. 1980;43:138–142.
- Busscher HJ, Rinastiti M, Siswomihardjo W, et al. Biofilm formation on dental restorative and implant materials. J Dent Res. 2010;89:657–665.
- Morgan TD, Wilson M. The effects of surface roughness and type of denture acrylic on biofilm formation by Streptococcus oralis in a constant depth film fermentor. J Appl Microbiol. 2001;91:47–53.
- Abuzar MA, Bellur S, Duong N, et al. Evaluating surface roughness of a polyamide denture base material in comparison with poly (methyl methacrylate). J Oral Sci. 2010;52:577–581.
- Serrano-Granger C, Cerero-Lapiedra R, Campo-Trapero J, et al. In vitro study of the adherence of Candida albicans to acrylic resins: relationship to surface energy. Int J Prosthodont. 2005;18:392–398.
- Dar-Odeh NS, Shehabi AA. Oral candidosis in patients with removable dentures. Mycoses. 2003;46:187–191.
- de Freitas Fernandes FS, Pereira-Cenci T, da Silva WJ, et al. Efficacy of denture cleansers on Candida spp. biofilm formed on polyamide and polymethyl methacrylate resins. J Prosthet Dent. 2011;105:51–58.
- Matthews E, Smith D. Nylon as a denture base material. Br Dent J. 1955;98:231–237.
- Ucar Y, Akova T, Aysan I. Mechanical properties of polyamide versus different PMMA denture base materials. J Prosthodont. 2012;21:173–176.
- Watt D. Clinical assessment of nylon as a partial denture base material. Br Dent J. 1955;98:238–244.
- Hargreaves AS. Nylon as a denture-base material. Dent Pract Dent Rec. 1971;22:122–128.
- Hamanaka I, Takahashi Y, Shimizu H. Mechanical properties of injection-molded thermoplastic denture base resins. Acta Odontol Scand. 2011;69:75–79.
- Sepulveda-Navarro WF, Arana-Correa BE, Borges CP, et al. Color stability of resins and nylon as denture base material in beverages. J Prosthodont. 2011;20:632–638.
- Takabayashi Y. Characteristics of denture thermoplastic resins for non-metal clasp dentures. Dent Mater J. 2010;29:353–361.
- Yunus N, Rashid AA, Azmi LL, et al. Some flexural properties of a nylon denture base polymer. J Oral Rehabil. 2005;32:65–71.
- Parvizi A, Lindquist T, Schneider R, et al. Comparison of the dimensional accuracy of injection-molded denture base materials to that of conventional pressure-pack acrylic resin. J Prosthodont. 2004;13:83–89.
- Fueki K, Ohkubo C, Yatabe M, et al. Clinical application of removable partial dentures using thermoplastic resin—Part I: definition and indication of non-metal clasp dentures. J Prosthodont Res. 2014;58:3–10.
- Fueki K, Ohkubo C, Yatabe M, et al. Clinical application of removable partial dentures using thermoplastic resin. Part II: material properties and clinical features of non-metal clasp dentures. J Prosthodont Res. 2014;58:71–84.
- ISO: Dentistry – Base polymers. Part 1: Denture base polymers. Art dentaire – Polymères de base. Partie 1: Polymères pour base de prothèses dentaires. 1st ed. Geneva, Switzerland: International Organization for Standardization, ISO 20795-1; 2008.
- Hamanaka I, Iwamoto M, Lassila L, et al. Influence of water sorption on mechanical properties of injection-molded thermoplastic denture base resins. Acta Odontol Scand. 2014;72:859–865.
- Bates JF, Stafford GD, Huggett R, et al. Current status of pour type denture base resins. J Dent. 1977;5:177–189.
- Singh RD, Gautam R, Siddhartha R, et al. High performance liquid chromatographic determination of residual monomer released from heat-cured acrylic resin. An in vivo study. J Prosthodont. 2013;22:358–361.
- Zissis A, Yannikakis S, Polyzois G, et al. A long term study on residual monomer release from denture materials. Eur J Prosthodont Restor Dent. 2008;16:81–84.
- Kopperud HM, Kleven IS, Wellendorf H. Identification and quantification of leachable substances from polymer-based orthodontic base-plate materials. Eur J Orthod. 2011;33:26–31.