Abstract
Aim: The aim of this study was to compile the usage of Co-Cr alloys in fixed prosthodontics (FP) among dental laboratories in Sweden.
Methods: From March to October 2015, questionnaires were sent to 542 registered dental laboratories in Sweden. The questionnaires were divided in two parts, one for fixed dental-supported prosthodontics (FDP) and one for fixed implant-supported prosthodontics (FIP). Reminders were sent three times.
Results: In total of 542 dental laboratories, 55% answered the questionnaires. Most dental laboratories use Co-Cr in FP, 134 (74%) in FDP and 89(66%) in FIP. The laboratories used Co-Cr alloys of various compositions in the prostheses, 35 for FDP and 30 for FIP. The most commonly used Co-Cr alloys for tooth-supported FDPs were (a) Wirobond® 280, (b) Cara SLM and (c) Wirobond® C. For implant-supported frameworks the frequently used alloys were: (a) Cara SLM, (b) Cara Milled and (c) Wirobond® 280. Except for the difference in composition of these alloys, they were also manufactured with various techniques. In tooth-supported prostheses the dominating technique was the cast technique while newer techniques as laser-sintering and milling were more commonly reported for implant-supported constructions. A fourth technique; the ‘pre-state’ milling was reported in FDP.
Conclusion: More than 30 different Co-Cr alloys were reported as being used in FP. Thus, there is a need for studies exploring the mechanical and physical behavior and the biological response to the most commonly used Co-Cr alloys.
Introduction
Gold has been the ‘gold standard’ material for replacing missing teeth with FDP due to desirable properties such as biocompatibility and ductility. However, due to high price and the request for alloys with better mechanical properties, new materials have entered the dental market [Citation1,Citation2].
In 1999, the Swedish National Board of Health and Welfare (Socialstyrelsen, www.socialstyrelsen.se) suspended a former regulation which prohibited base-metal alloys in FDPs for permanent use because of the risk of hypersensitivity induced by nickel, cobalt and chromium [Citation3]. Until then, the use of base metal alloys was only allowed for removable dental prostheses (RDPs) or temporary FDPs. Yet, the alloys for porcelain-fused-to-metal prostheses differ globally; nickel-chromium alloys (Ni-Cr) are mostly used in the USA, in contrast to Sweden and Japan where cobalt-chromium alloys (Co-Cr) are more commonly used [Citation1].
The advantages with Co-Cr for dental use are the mechanical properties, i.e. stiffness (high elastic modulus) that render a possibility for reduced dimension of the framework [Citation4] and adequate bond strength between the porcelain and alloy [Citation5] and corrosion resistance [Citation6]. Disadvantages are the markedly higher corrosion in acidic environments, extended chair side time needed for finishing and polishing because of the hardness and casting difficulties [Citation1]. Another disadvantage is the limited knowledge of the longevity of Co-Cr alloys in fixed prosthodontics [Citation7]. Furthermore, there have been concerns related to the biocompatibility of CoCr [Citation8,Citation9]. Another concern related to CoCr is the risk for the dental technician to inhale grinding dust, during adjustments and polishing [Citation10].
Besides new materials, novel manufacturing techniques, such as subtractive manufacturing; milling, ‘pre-state’ milling [Citation11] and additive manufacturing; DMLS (Direct Metal Laser Sintering) [Citation12], SLS (Selective Laser Sintering), SLM (Selective Laser Melting) have also entered the dental market, and partly replaced the traditional casting technique. Despite sparse clinical evaluation these techniques are applied to Co-Cr and are currently increasingly used in fixed prosthodontics [Citation12,Citation13]. However, in vitro studies of these new manufacturing techniques show different material properties such as fit [Citation11,Citation14–19] and metal release [Citation20] but similar porcelain adhesion [Citation21,Citation22] and surface hardness [Citation23].
In Sweden, all prosthetic devices are regulated by legislation regarding medical devices from the Swedish Medical Products Agency, MPA (Läkemedelsverket, https://lakemedelsverket.se), an agency which also requires registration of all dental laboratories [Citation24]. Dental laboratories (manufacturers) are obliged to have Material Safety Datasheets (MSDS) available for every custom-made medical device manufactured for each patient. According to the regulations [Citation25], the MSDS shall contain the following headings: (1) identification of the substance/preparation and of the company/undertaking, (2) hazards identification, (3) composition/information on ingredients, (4) first-aid measures, (5) fire-fighting measures, (6) accidental release measures, (7) handling and storage, (8) exposure controls/personal protection, (9) physical and chemical properties, (10) stability and reactivity and (11) toxicological information.
However, no specific clinical evaluation is required by the Swedish MPA. Further, as mentioned earlier, clinical studies regarding various Co-Cr alloys are sparse [Citation7]. Thus, more research is needed to investigate which Co-Cr alloys are used in dentistry today and how they are manufactured.
Aim
The aim of the present study was to conduct a survey among the dental laboratories in Sweden and specifically address and compile the usage of Co-Cr alloys for fixed dental prosthodontics according to composition and fabrication technique.
Materials and methods
In spring 2015 the Swedish MPA was contacted regarding all the dental laboratories registered in Sweden. A total of 542 dental laboratories were defined and included in the study.
Two surveys with six questions each, one for FDP and one for FIP, were prepared. Two local dental laboratories were asked for feed-back regarding the content in the questionnaires. The final questionnaires included a total of 20 questions and were divided in two parts, ().
Table 1. Questionnaires: Two questionnaires containing two parts; one for tooth (Part I)- and one for implant-supported fixed prosthodontics (Part II).
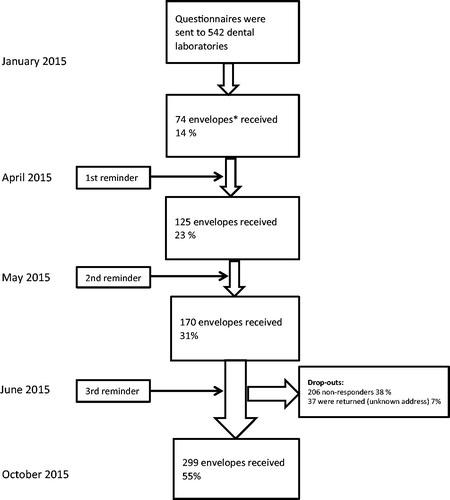
The surveys were sent by post, to the 542 dental laboratories and reminders were sent three times. Due to the low frequency of answers, the Swedish Association of Dental Technicians (Sveriges Tandteknikerförbund) was contacted and a small item about the ongoing survey was published at their website, www.dentallab.se, explaining the project and encouraging the members to answer. Because of low answer frequency, 23%, after the first reminder, one initial question defining working tasks was added in each questionnaire, (). After a third reminder, the frequency of answers increased from 23 to 55%. Data collection was completed in October 2015, see flow-chart, (). For data registration and calculation IBM SPSS version 20 and Microsoft Excel 2010 were used.
Figure 1. Flow chart for the study showing the total number of questionnaires distributed to 542 dental laboratories; the number of reminders; the number of drop-outs and the total number of envelopes received. *Each dental laboratory received one envelope containing: (a) A letter describing the study. (b) Two questionnaires containing two parts; one for tooth (Part I) – and one for implant-supported fixed prosthodontics (Part II). (c) Return envelope with postage included.
Results
From the 542 sent envelopes, 37 (7%) were returned because of unknown address, and 206 (38%) were non-responders. In total, 299 (55%) answers were received, (). Results could only be extracted from the first 5 questions (1–5), because of a high drop-out in answers from the questions 6–10. Moreover, 22 registered dental laboratories were not relevant for the study; one dental laboratory (0.3%) was taken-over by another company, 5 (2%) were not dental technicians, e.g. dentists with chairside production or mother-companies, 5 (2%) were subsuppliers and 11 (4%) were inactive. One (0.3%) dental laboratory reported that they had their production abroad without providing any further information. There were differences in the drop-out rates between the two questionnaires. For the FDP questionnaires the drop-out reached 5% while the drop-out in the FIP part reached 14%. Results from the total 299 received answers demonstrated that 180 (60%) dental laboratories worked with FDP in comparison with 135 (45%) for FIP, (). Additionally, 86 (29%) dental laboratories worked with FDP and FIP. Forty-eight (16%) exclusively worked with FDP and 3 (1%) with FIP.
Table 2. Answers from 299 received questionnaires concerning tooth-supported and implant-supported constructions.
From the 181 laboratories that answered accounted for choice of material in FDPs, 138 (76%) use zirconium dioxide, 135 (75%) use Co-Cr, 84 (46%) high-noble gold, 64 (35%) titanium (both Commercially Pure, CP and alloys) and 105 (58%) other materials such as all-ceramics or other alloys, ().
Figure 2. Question 2. For tooth-supported, which metal/alloy/ceramics does your laboratory use? For implant-supported, which metal/alloy/ceramics does your laboratory use? *Other: Noble-alloys, palladium-alloys and other ceramics.
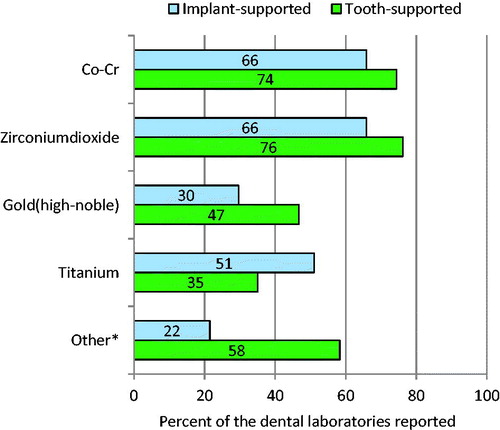
In addition, the answers from 139 dental laboratories in FIP indicated that 91 laboratories (66%) use Co-Cr and 90 (64%) use zirconium dioxide, 72 (52%) use titanium (both Commercially Pure and alloys), 40 (29%) use gold and 29 (21%) use other materials such as all-ceramics and other alloys, ().
In total, 86 (29%) dental laboratories work with both FDP and FIP. Forty-eight (16%) exclusively worked with FDP and three (1%) exclusively with FIP. Furthermore, the results showed that a higher number of dental laboratories worked with FDP than with FIP, 180 (60%) and 135 (45%) respectively, ().
Regarding type of titanium, the results revealed that 59% of the dental laboratories used titanium grade 1–4 and 30% used a titanium alloy in the FDP group. In the FIP group, 70% used titanium grade 1–4 and 35% used titanium alloy.
Finally, 134 (45%) and 89 (30%) dental laboratories that reported the use of Co-Cr alloys in FDP and FIP, respectively, were included ().
FDP
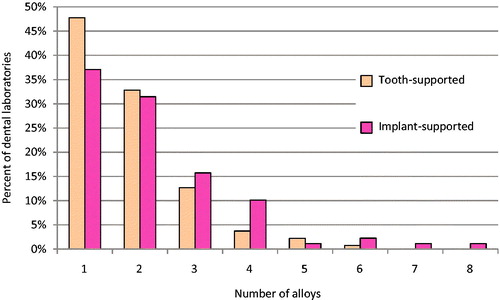
The results showed that the 134 dental laboratories that were included in the study use 35 different Co-Cr alloys, (). The most commonly used alloys were (1) Wirobond® 280, (2) Cara SLM, (3) Wirobond® C, (4) Remanium® Star CL, and (5) Solibond C plus and (6) Heranium PW. The total number of unknown alloys was 5 and was registered by 18% of the dental laboratories. Moreover, 4% of the dental laboratories register 2 unknown alloys and 14% registered 1 unknown alloy. Of the totally 5 unidentified alloys, 4 could not be found on the internet and one was registered as ‘Wirobond’ without further specification.
Figure 3. Co-Cr alloys in tooth-supported fixed prosthodontics. Results from the Swedish survey 2017 indicate that dental laboratories use 35 Co-Cr alloys in fixed tooth-supported prosthodontics. Almost 18% of the dental laboratories registered alloys that could not be identified.
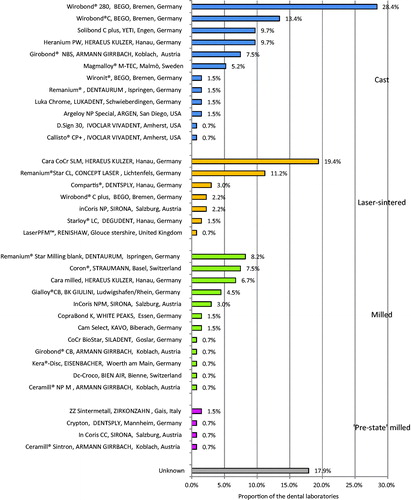
The prostheses were manufactured by four different techniques; casting, milling, SLM and milling/sintering, (). The reported Co-Cr alloy of choice were related to the manufacturing technique; Cast: (1) Wirobond® 280, (2) Wirobond® C, (3) Solibond C plus and Heranium PW. Milled: (1) Remanium® Star Milling Blank, (2) Coron® and (3) Cara SLM. SLM: (1) Cara SLM, (2) Remanium® Star CL and (3) Compartis.
‘Pre-state’ milling (1) Zirkonzahn Zintermetal and (2) Ceramill Sintron, In Coris CC Sirona and Crypton.
The dental laboratories use one up to six different Co-Cr alloys, ().
FIP
The results demonstrated that the 89 dental laboratories that were included in the study use 30 different Co-Cr alloys (). A majority of the dental laboratories use (1) Cara SLM 27%, (2) Cara Milled 26%, (3) Wirobond® 280 19%, (4) Wirobond® C 14%, (5) Coron® 12%, and (6) Remanium® Star CL 11%. As shown in the element composition of these alloys differs. Unknown alloys were registered by 36% of the dental laboratories. In detail, there were 2% of the dental laboratories that registered 3 unknown alloys, 7% registered 2 unknown alloys and 15% registered 1 unknown alloy. Nine alloys were registered as unknown. Out of theses, 6 alloys could not be identified and 3 were registered as ‘Wirobond’, ‘Scheftner’ and ‘Dentaurum’ without further specification.
Figure 5. Co-Cr alloys in implant-supported fixed prosthodontics. Results from the Swedish survey 2017 indicate that dental laboratories use 30 Co-Cr alloys in fixed implant-supported prosthodontics that are manufactured by three different techniques (laser-sintered, milled and cast). Almost 36% of the dental laboratories registered alloys that could not be identified.
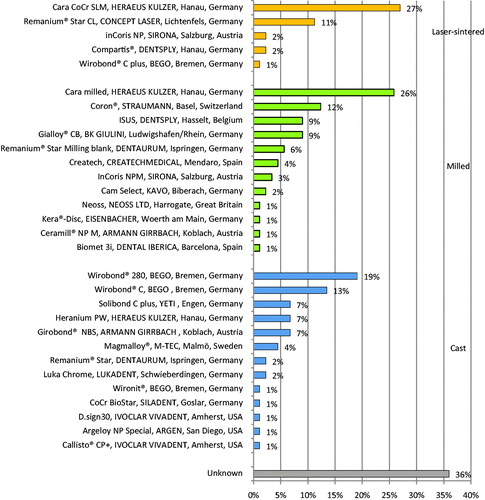
Table 3. The composition of the most reported Co-Cr alloys.
Moreover, the alloys were manufactured by three different techniques; casting, milling and SLM ().
The most reported Co-Cr alloys depended on manufacturing technique were; SLM: (1) Cara SLM, (2) Remanium® Star CL and (3) inCoris NP. Milled: (1) Cara SLM (2) Coron® (3) ISUS. Cast: (1) Wirobond® 280, (2) Wirobond® C, and (3) Solibond C plus, (4) Heranium PW and (5) Girobond® NBS. The dental laboratories used up to eight different Co-Cr alloys ().
The element composition of the most commonly reported Co-Cr alloys varied in both tooth- and implant-supported fixed prosthodontics as can be seen in .
Discussion
This paper presents a survey on the usage of dental materials and specifically on Co-Cr alloys, used in FP in Sweden between January and October 2015. The results showed that dental laboratories use 35 and 30 different Co-Cr alloys in FDP and FIP, respectively, manufactured by 3–4 different techniques ( and ).
The response rates in other similar studies vary between 32 and 76% [Citation26–32]. A higher response rate in the study by Baumann et al., of 76% could be explained by the initial inclusion process where questionnaires were sent to dental laboratories that advertised in the Yellow Pages Group of New Zealand excluding the laboratories that only advertised removable prostheses. And the non-responders were contacted by phone which probably increased the answering frequency more. In accordance to the present study, Baumann et al. sent the questionnaires by post due to the fact that some dental laboratories indicated the absence of an email-address [Citation30].
The present survey revealed that 22 (7%) registered dental laboratories were not relevant for the study. Furthermore, 1 (0.3%) dental laboratory reported that they had their production abroad. Nonetheless, did Alameri et al. identify that 14 (22%) of the dental laboratories in New Zealand used offshore laboratories [Citation26]. The difference may be explained by demographical factors i.e. New Zealand having fewer dental laboratories compared to their needs or that the dental laboratories in the present survey underreported the extent of the abroad production.
Results from the present study show that more dental laboratories work with FDP than FIP, 60 and 45% respectively, (). Similar findings were reported by Afsharzand et al [Citation31,Citation32], i.e. more dental laboratories in the U.S worked with FDP than FIP, yielding a frequency rate of 47 and 36%, respectively. Baumann et al. found that 42% of the dental laboratories in New Zealand worked with FP [Citation30].
Most dental laboratories in the present study use Zirconium dioxide and Co-Cr, 76% and 75%, respectively, in FDP while high-noble gold is used by 46%, titanium (both CP and alloys) by 35% and other materials as palladium-based alloys or other all-ceramics by 58% of dental laboratories (). Results from another survey by Berry et al. demonstrated that 52% of the dental laboratories used base-metal alloy (without further information) as crown material [Citation29]. In addition, the same authors report that dental laboratories used all-ceramic in 29% of the anterior cases and 8% in the posterior cases [Citation29]. The same study reported that 8% used high-gold content alloy. Higher frequency of noble-alloy use could be seen in New Zealand [Citation30] where dental laboratories used 55 different alloys out of which 35 (64%) were high-noble, 13 (23%) were noble and 7 (13%) were base-metal alloys. Another survey from New Zealand [Citation26] showed that all-metal and porcelain-fused-metal (PFM) alloys (76%) were the most common material used in fixed prosthodontics, followed by titanium (57%). Most of the studies that describe metal ceramic constructions refer to base-metal alloy or PFM (porcelain-fused metal) without further information [Citation29,Citation33,Citation34]. Thus, comparisons of results between these studies are difficult.
In the present survey, the results show a higher use of Zirconium dioxide and Co-Cr in FDP, compared to other studies [Citation26,Citation29,Citation30]. This could eventually be explained by the time factor in this survey, being the most recent.
As can be seen, there is a clear difference in the use of materials in FP between FDP and FIP. Co-Cr and Zirconium dioxide dominates but in a lower frequency in FIP, while titanium is the third most commonly used material in FIP compared to gold in FDP. The biocompatibility of titanium as a material supported prostheses for implants is well known and documented [Citation35,Citation36], while there has been some concerns regarding Co-Cr [Citation8]. Hagiwara et al. sent questionnaires to 120 out of 285 certified dental laboratories in Japan and identified that PFM was the most commonly reported material used for FIP in the anterior and posterior region, 43% and 31%, respectively, followed by Zirconia 27% and 14%, respectively [Citation37]. These results must be interpreted with caution because of the high exclusion rate in that study, which could affect the outcome as well as the earlier mentioned difficulty with inadequate material information.
According to the present study, the most commonly used CoCr-alloys were Wirobond® 280 (cast) for FDP, and Cara SLM for FIP. This could be explained by one of the advantages of AM (additive manufacturing); its ability to create parts with complex morphology, both external and internal [Citation38], as implant frameworks, and also with reduced energy consumption [Citation39]. Besides that does the cooling rate in AM create parts with reduced grains [Citation40]. Gold casts with fine grain size have generally superior mechanical properties, i.e. as tensile strength and elongation compared to casts with rough grain size [Citation41]. Similarly, Co-Cr alloys with smaller grain size show better mechanical properties; as higher strength and ductility when grain sizes were smaller [Citation42]. Furthermore, in a study by Joda and Brägger it was shown that AM facilitates the production process with an 18% cost benefit when digital flow was compared with conventional flow [Citation43].
Another advantage of AM compared to milling is lower waste of the material needed [Citation39]. Results from the present study showed that the Co-Cr alloys used in implant prosthodontics were milled inwards towards the implant surface. Siemieniuch et al. mentioned that materials manufactured by AM showed an imprecise surface which needed further milling [Citation39]. Results from the literature regarding fit between Co-Cr alloys manufactured by different ways show contradictory results [Citation14–16]. Reasons for that could be (a) a difference between the materials per se or (b) that different measuring techniques are used that makes the results difficult to compare and (c) the absence of consensus of an acceptable fit.
The present study showed that the dental laboratories frequently did not register neither the name of the Co-Cr alloy nor the manufacturing technique, but only the manufacturer name. The datasheets that were obtained from the manufacturers or by searching the internet did not contain information about the recommended manufacturing technique. Moreover, datasheets for some Co-Cr alloys were not possible to find.
During the time from the data registration to the analysis, two alloys, Crypton (‘pre-state’ milling) and Cam Select (cast) were not available any longer. This shows how quickly materials can be implemented and phased out before they are sufficiently evaluated. Thus, insufficient knowledge of Co-Cr alloys in combination with the wide range of available alloys that was shown in the present study is a challenge for the dentist who is responsible for the prosthesis delivered to the patient.
Conclusions
Within the limitations of the present study the following conclusions could be made:
Co-Cr alloys were more frequently used in FDP than in FIP.
Dental laboratories report that they use 35 and 30 different Co-Cr alloys in FDP and FIP.
Three different techniques were reported in both FDP and FIP; cast, milling and laser-sintering, while one additional technique; ‘pre-state’ milling was only reported in FDP.
Cast frameworks were more common in the FDPs and laser-sintering and milling were more common in the FIPs.
There is a need for further studies investigating possible differences between the most commonly reported Co-Cr alloys in order to provide for patient safety.
Acknowledgements
The authors wish to thank all dental laboratories who participated in this survey and the Swedish Association of Dental Technicians for their valuable help in highlighting the survey on their web-site.
The present study has been supported by Futurum – the Academy for health and care, Region JönkÖping County, Sweden, the Wilhelm and Martina Lundgren Research Foundation, Sweden, the Hjalmar Svensson Research Foundation, Sweden and the Sylvan Foundation, Sweden.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Wataha JC. Alloys for prosthodontic restorations. J Prosthet Dent. 2002;87:351–363.
- Anusavice KJ, Phillips RW, Shen C, et al. Chapter 4, Indirect restorative materials. In: Phillips' science of dental materials. 12nd ed. St. Louis(MO): Elsevier/Saunders; 2013. p. 368.
- Bessing C. Oädla legeringar för metallkeramik: Basmetallegeringar [Base metal alloys for porcelain-fused constructions: Base metal alloys]. 2.0 ed. Stockholm: Socialstyrelsen, Kunskapscenter för Dentala Material, 2007 [Swedish].
- Anusavice KJ. Chapter 19, Dental casting and soldering. In: Phillips' science of dental materials. St. Louis (MO): Saunders; 2003. p. 596.
- Joias RM, Tango RN, Junho de Araujo JE, et al. Shear bond strength of a ceramic to Co-Cr alloys. J Prosthet Dent. 2008;99:54–59.
- Holm C, Morisbak E, Kalfoss T, et al. In vitro element release and biological aspects of base–metal alloys for metal-ceramic applications. Acta Biomater Odontol Scand. 2015;1:70–75.
- Svanborg P, Langstrom L, Lundh RM, et al. A 5-year retrospective study of cobalt-chromium-based fixed dental prostheses. Int J Prosthodont. 2013;26:343–349.
- Hjalmarsson L, Smedberg JI, Aronsson G, et al. Cellular responses to cobalt-chrome and CP titanium-an in vitro comparison of frameworks for implant-retained oral prostheses. Swed Dent J. 2011;35:177–186.
- Arvidson K, Cottler-Fox M, Hammarlund E, et al. Cytotoxic effects of cobalt-chromium alloys on fibroblasts derived from human gingiva. Eur J Oral Sci. 1987;95:356–363.
- Selden AI, Persson B, Bornberger-Dankvardt SI, et al. Exposure to cobalt chromium dust and lung disorders in dental technicians. Thorax. 1995;50:769–772.
- Vojdani M, Torabi K, Atashkar B, et al. A comparison of the marginal and internal fit of cobalt- chromium copings fabricated by two different CAD/CAM Systems (CAD/Milling, CAD/Ceramill Sintron)). J Dent (Shiraz). 2016;17:301–308.
- Barazanchi A, Li KC, Al-Amleh B, et al. Additive technology: update on current materials and applications in dentistry. J Prosthodont. 2017;26:156–163.
- Hjalmarsson L. Kobolt-krom eller titan? [Cobalt-chromium or titanium?]. Tandläkartidningen. 2013;105:64–67. [Swedish].
- Ortorp A, Jonsson D, Mouhsen A, et al. The fit of cobalt-chromium three-unit fixed dental prostheses fabricated with four different techniques: a comparative in vitro study. Dent Mater. 2011;27:356–363.
- Ucar Y, Akova T, Akyil MS, et al. Internal fit evaluation of crowns prepared using a new dental crown fabrication technique: laser-sintered Co-Cr crowns. J Prosthet Dent. 2009;102:253–259.
- Kim EH, Lee DH, Kwon SM, et al. A microcomputed tomography evaluation of the marginal fit of cobalt-chromium alloy copings fabricated by new manufacturing techniques and alloy systems. J Prosthet Dent. 2017;117:393–399.
- Svanborg P, Skjerven H, Carlsson P, et al. Marginal and internal fit of cobalt-chromium fixed dental prostheses generated from digital and conventional impressions. Int J Dent. 2014;2014:534382.
- Huang Z, Zhang L, Zhu J, et al. Clinical marginal and internal fit of metal ceramic crowns fabricated with a selective laser melting technology. J Prosthet Dent. 2015;113:623–627.
- Quante K, Ludwig K, Kern M. Marginal and internal fit of metal-ceramic crowns fabricated with a new laser melting technology. Dent Mater. 2008;24:1311–1315.
- Hedberg YS, Qian B, Shen Z, et al. In vitro biocompatibility of CoCrMo dental alloys fabricated by selective laser melting. Dental Mater. 2014;30:525–534.
- Serra-Prat J, Cano-Batalla J, Cabratosa-Termes J, et al. Adhesion of dental porcelain to cast, milled, and laser-sintered cobalt-chromium alloys: Shear bond strength and sensitivity to thermocycling. J Prosthet Dent. 2014;112:600–605.
- Lawaf S, Nasermostofi S, Afradeh M, et al. Comparison of the bond strength of ceramics to Co-Cr alloys made by casting and selective laser melting. J Adv Prosthodont. 2017;9:52–56.
- Xin XZ, Chen J, Xiang N, et al. Surface properties and corrosion behavior of Co-Cr alloy fabricated with selective laser melting technique. Cell Biochem Biophys. 2013;67:983–990.
- Tandtekniska arbeten: en vägledning till reglerna om medicintekniska produkter.[Dental laboratory products: a guide of the regulation of medical devices]. Läkemedelsverket [Medical Products Agency]. Stockholm: Läkemedelsverket; 2011. [Swedish].
- European Agency for Safety and Health at Work. Regulation (EC) No 1907/2006; 2006 [cited 2017 April 20]. p.124–165. Available from: http://data.europa.eu/eli/reg/2006/1907/2014-04-10
- Alameri SS, Aarts JM, Smith M, et al. Dental technology services and industry trends in New Zealand from 2010 to 2012. N Z Dent J. 2014;110:65–73.
- Bower EJ, Newton PD, Gibbons DE, et al. A national survey of dental technicians: career development, professional status and job satisfaction. Br Dent J. 2004;197:144–148.
- Berry J, Nesbit M, Saberi S, et al. Communication methods and production techniques in fixed prosthesis fabrication: a UK based survey. Part 1: communication methods. Br Dent J. 2014;217:E12.
- Berry J, Nesbit M, Saberi S, et al. Communication methods and production techniques in fixed prosthesis fabrication: a UK based survey. Part 2: production techniques. Br Dent J. 2014;217:E13.
- Baumann B, Pai WH, Bennani V, et al. Dental alloys used for crown and bridge restorations by dental technicians in New Zealand. N Z Dent J. 2010;106:43–49.
- Afsharzand Z, Rashedi B, Petropoulos VC. Dentist communication with the dental laboratory for prosthodontic treatment using implants. J Prosthodontics. 2006;15:202–207.
- Afsharzand Z, Rashedi B, Petropoulos VC. Communication between the dental laboratory technician and dentist: work authorization for fixed partial dentures. J Prosthodont. 2006;15:123–128.
- Pjetursson BE, Sailer I, Makarov NA, et al. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part II: Multiple-unit FDPs. Dent Mater. 2015;31:624–639.
- Sailer I, Makarov NA, Thoma DS, et al. All-ceramic or metal-ceramic tooth-supported fixed dental prostheses (FDPs)? A systematic review of the survival and complication rates. Part I: Single crowns (SCs). Dent Mater. 2015;31:603–623.
- Branemark PI. Osseointegration and its experimental background. J Prosthet Dent. 1983;50:399–410.
- Adell R, Lekholm U, Rockler B, et al. A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 1981;10:387–416.
- Hagiwara Y, Narita T, Shioda Y, et al. Current status of implant prosthetics in Japan: a survey among certified dental lab technicians. Int J Implant Dent. 2015;1:4.
- Zandparsa R. Digital imaging and fabrication. Dent Clin North Am. 2014;58:135–158.
- Siemieniuch CE, Sinclair MA, Henshaw MJ. Global drivers, sustainable manufacturing and systems ergonomics. Appl Ergon. 2015;51:104–119.
- Frazier WE. Metal additive manufacturing: a review. J F Materi Eng Perform. 2014;23:1917–1928.
- Nielsen JP, Tuccillo JJ. Grain size in cast gold alloys. J Dent Res. 1966;45:964–969.
- Vajpai SK, Sawangrat C, Yamaguchi O, et al. Effect of bimodal harmonic structure design on the deformation behaviour and mechanical properties of Co-Cr-Mo alloy. Mater Sci Eng C Mater Biol Appl. 2016;58:1008–1015.
- Joda T, Bragger U. Digital vs. conventional implant prosthetic workflows: a cost/time analysis. Clin Oral Implants Res. 2015;26:1430–1435.