Abstract
Objective: Oral rehabilitation success is enhanced by an accurate and reproducible final impression. The purpose of this study is to evaluate the dimensional changes of a polyether and addition silicone subjected to disinfection and/or sterilization after a long storage period.
Material and methods: Ninety samples were obtained from polyether ImpregumTM PentaTM (3M ESPETM, Seefeld, Germany) and 90 of addition silicone ImprintTM 4 PentaTM Putty (3M ESPETM, Seefeld, Germany) according to ISO 4823:2000. The samples of each material were split to form three groups with 30 samples each: a control group, a hypochlorite group (disinfection) and an autoclave group (sterilization). Samples were stored in the Portuguese Institute for Quality for six months at 23 °C. Samples were measured by laser interferometry, according to the Michelson technique before calculating dimensional stability according ISO 4823:2000. A statistical analysis via a three-way mixed ANOVA was performed.
Results: Significant shrinkage of ImpregumTM PentaTM was 0.77 ± 0.17% in the control group, 0.42 ± 0.19% in the hypochlorite group and 0.52 ± 0.28% in the autoclave group. For ImprintTM 4 PentaTM Putty, the control group had a shrinkage of 0.42 ± 0.12%, the hypochlorite group 0.36 ± 0.09% and the autoclave group 0.59 ± 0.13%.
Conclusions: The long-term storage of samples subjected to disinfection with 5.25% hypochlorite or autoclave sterilization can be used in a clinical setting as the dimensional changes are below the maximum permitted by the ISO 4823:2000, since there are no clinically significant changes in the dimension of the samples during the storage period.
Introduction
Impression materials are presently still a relevant material for use in restorative dentistry [Citation1–3]. Impressions are used to transfer the information from the patient’s mouth to a stone analog cast, which can aid in making a diagnosis and a correct treatment plan, critical to the success of final prosthetic restoration [Citation4].
The impression material selected by the dentist must provide good dimensional stability and precision in detail reproduction. The material should not suffer changes during the disinfection or sterilization processes and should allow adequate storage stability over time [Citation5,Citation6].
Elastomers are the most commonly used impression material in dentistry [Citation1]. Within this group, polyethers (PE) and addition silicones/vinyl polysiloxanes (VPS) exhibit excellent dimensional stability against distortion under various storage conditions [Citation6]. Several authors demonstrated that there are differences between the two materials [Citation4,Citation7,Citation8]. Unlike VPS, PE has an hydrophilic nature, which can lead to the absorption of water [Citation4,Citation7,Citation8], and the casting of this material should be conducted 1 h after making the impression [Citation7–9]. The VPS may be cast immediately after removal from the oral cavity or weeks after completing the impression, since it is not susceptible to moisture changes, and there are no products derived from the polymerization reaction [Citation5,Citation9–13]. VPS material exhibits better reproduction of detail and greater dimensional long-term stability when compared to PE [Citation4,Citation5,Citation9–13].
Impression materials come into contact with potential sources of contamination, such as blood and saliva that might contain pathogens [Citation14]. Thus, to prevent transmission of infectious diseases (hepatitis B, hepatitis C, human immunodeficiency virus, herpes, tuberculosis), it is fundamental to perform cross-infection control procedures, such as sterilization and disinfection, by dental practices and laboratories [Citation3,Citation15,Citation16].
Although there are several disinfection methods proposed, chemical disinfection of impressions by immersing in a disinfectant solution is considered the most practical and reliable way [Citation6,Citation17]. The ADA recommends that the molds must be disinfected by soaking with compatible products [Citation18]. The selected disinfectant solution should demonstrate high effectiveness in the reduction of pathogenic microorganisms without interfering with the dimensional stability or ability to reproduce details of the material [Citation1,Citation16].
Unlike disinfection, sterilization is a procedure that guarantees the elimination of all microorganisms [Citation19]. There is no universally accepted method of sterilization, but the literature suggests that the autoclave is considered the most effective method [Citation6,Citation18], although its effects on the dimensional stability of the elastomeric impression materials are not sufficiently described in the literature [Citation20].
After disinfection or sterilization, the impressions are cast in stone. The dimensional stability of the impression materials depends on the time elapsed between the completion of the impression and their casting, thus storage time is critical to obtain reliable casts [Citation21].
Several authors have studied impressions after disinfection or sterilization [Citation22–29] without a clear agreement. Some claim disinfection and sterilization has no adverse effect on the dimensional stability of impressions [Citation15,Citation24,Citation30], while others point to possible adverse effects [Citation25,Citation31].
Walker et al. [Citation32] pioneered the development of studies on the dimensional stability of long-term impression materials, introducing the variable disinfection and sterilization of materials. Her research proposed a maximum storage time of silicones for 2 weeks; however, the literature suggests that impressions can be reused weeks or months after the setting of the material [Citation2,Citation32,Citation33]. Thus, the present study seems to be innovative and appropriate, since there are no studies correlating a 6-month storage time along with the variables of disinfection and sterilization.
The objective of this study is to evaluate the dimensional changes of a VPS and a PE after disinfection and sterilization after a 6-month storage period. The null hypothesis is that there is no dimensional change of the materials studied, while the alternative suggests there is dimensional change in the materials studied after disinfection and after sterilization after a 6-month storage period.
Materials and methods
For this study, ImprintTM 4 PentaTM Putty Impression Material (3M ESPETM, Seefeld, Germany, Lot 549538) as a VPS and as a PE, ImpregumTM PentaTM (3M ESPETM, Seefeld, Germany, Lot 559739).
The samples were obtained following ADA specification No 19 and ISO 4823:2000 protocol [Citation34]. This ISO specifies the use of a block test, a specific metallic cylindrical matrix/block test and a metal ring/ring mold ( and ). Both metal pieces were washed with deionized water in ultrasound for two cycles and then placed in an oven at 37 °C for 15 min prior to sample producing.
Figure 2. Superior view of the block test. Lines 1 and 2 have a width of 50 and 20 microns, respectively. Lines 3, 4 and 5 have a width of 75.
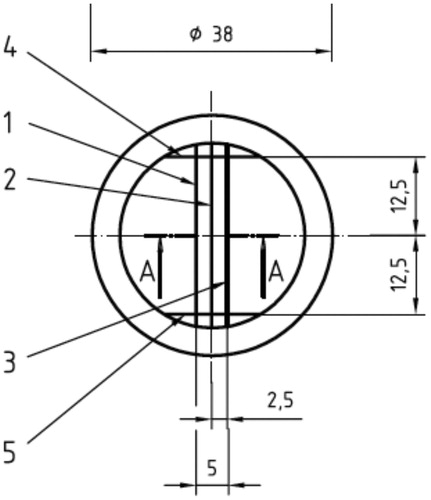
The VPS and PE were manipulated using the automatic mixer Pentamix 2 (3M ESPETM, Seefeld, Germany) in accordance with the manufacturer’s instructions. The obtained mixture was dispensed into the ring-matrix assembly. A rigid metal plate covered with a sheet of ethylene, was subsequently placed over the assembly, to ensure firm sealing of the material in the metal matrix. A two kilogram weight was placed on the sheet covering the metal plate so that the material was subjected to a constant force during the setting and in order to mimic the strength of the operator while making an impression. The entire assembly was immersed in a water bath at 35 °C to mimic the temperature of the oral cavity. To the setting time indicated by the manufacturer for both the PE (3:15 min) VPS (2:30 min), 3 min were added to ensure complete polymerization of both materials.
After the setting of the materials, they were removed from the bath. The samples were separated from the matrix, labeled, washed and dried with blown air. In order to approve a sample for testing, all samples were observed under a 4× magnifying glass Leica Stereo Zoom (Leica Microsystems, Wetzlar, Germany) by a single calibrated operator, in order to verify the continuity of the 75 micron line. If there was a continuity of the line, the sample was accepted.
Ninety (90) samples of each material were obtained and randomly distributed in three groups:
Sodium hypochlorite at 5.25% group: the samples were subjected to chemical disinfection by immersion in a sodium hypochlorite solution at 5.25% for 10 minutes.
Autoclave group: the samples were subjected to an autoclave sterilization protocol in a 40-minute cycle at 134 °C.
Control group: the samples were not subject to any type of sterilization or disinfection protocol.
All samples were measured twice, after sterilization or disinfection (T0) and after a 6-month storage (T1). Control group samples were measured after being obtained and at T1. The measurement protocol followed the guidelines of ISO 4823:2000. A Stemi 2000-C stereomicroscope (Carl Zeiss, Oberkochen, Germany) with a 12× magnification cross hair reticle, equipped with a XY table (only used for stabilizing the sample).
These instruments were mounted on cart that runs on a SIP3002M (Société Genevoise D`Instruments de Physiques, Geneva, Switzerland) rail. The cart also has a mirror to reflect the laser beam.
As the cart moves horizontally on the rail, it allows for all measurements to be made on the same axis with a HP® 5508 A (HewlettPackard, Santa Clara, CA) Michelson interferometer with an accuracy of 10 nm.
For each sample, the distance from the vertical lines 4 and 5 was measured three times for each horizontal line (1, 2, and 3), to ensure reproducibility of the method (), making a total of nine measurements in each sample. The same measurement method was applied to the mold before the impressions were made in order to calculate the dimensional change. The percentage of dimensional change for each specimen was calculated according to the formula presented by ISO 4823:2000:
where L1 represents the distance measured between the two vertical lines (4 and 5) on the mold and L2 represents the distance measured between the vertical lines (4 and 5) on the samples.
The samples were stored in the metrology department of the Portuguese Institute of Quality at 20 ± 2 °C at 70% humidity.
The data were introduced to IBM SPSS Statistics software – version 20.0. (IBM SPSS Statistics, Armonk, NY) A statistical analysis via three-way mixed ANOVA was performed. The presence of statistically significant differences or statistically significant interaction between groups is accepted in accordance with the level of significance p < .05.
Results
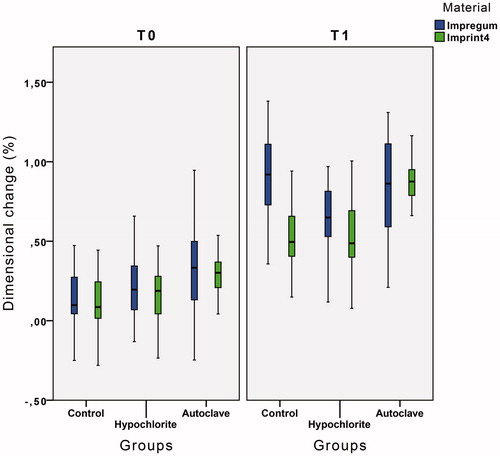
From the 180 samples, a total of 3240 measurements were recorded, 1080 for each group. Average dimensional change of PE and VPS samples in all groups at the two measurement times can be found in . At T0, the autoclave group has the highest average dimensional change (0.30 ± 0.30% for PE and 0.30 ± 0.12% for VPS) and the control group has the lowest average dimensional change (0.13 ± 0.19% for PE and 0.12 ± 0.18% for VPS). In T1, the hypochlorite group has the lowest average dimensional change for both materials (0.64 ± 0.23% for PE and 0.52 ± 0.24% for VPS). In T1 for the PE, the control group has the highest average dimensional change (0.90 ± 0.26%), and for the VPS, the autoclave group is the group that has the highest dimensional change (0.87 ± 0.13%).
Figure 3. Descriptive statistics of PE and VPS groups with mean values and standard deviation at T0 and T1 time level.
A three-way mixed ANOVA was run to understand the effects of material, studied groups and time on dimensional stability. There was a statistically significant three-way interaction between time, material, and studied groups (p < .001).
There was a statistically significant simple two-way interaction of material and groups at the T1 level (p < .025), but not at the T0 level (p = .794). That interaction arises from the statistically significant differences between the control groups of the two materials (p < .05). Statistical significance of a sample main effect was accepted at a Bonferroni-adjusted alpha level of 0.025.
According to sample main effects in the autoclave and hypochlorite groups, after the 6 months of storage, there were no significant differences in the dimensional behavior between two materials (p > .025). So, the PE and VPS when subjected to sterilization or disinfection do not differ in their dimensional behavior after a 6 months storage of the samples, but when no cross-infection control procedure is applied PE (0.90 ± 0.26%) presents higher shrinkage when compared to VPS (0.53 ± 0.19%) ().
Table 1. Descriptive and three-way ANOVA analysis between the three variables.
According to the sample comparisons, VPS control and VPS hypochlorite are different from VPS autoclave (p < .05) at T1 time level. A statistically significant difference (p < .05) of PE control and PE autoclave with PE hypochlorite was also found at T1 ().
There are statistically significant differences between the two measurement times in all groups for both materials, p < .05 (). Thus, all the PE and VPS groups had significant dimensional changes of the samples during their storage period. In the PE, the group that experienced smaller dimensional changes was the hypochlorite, with a statistically significant shrinkage of 0.42 ± 0.19%, the autoclave group and control groups where there were greater dimensional changes of the samples, with a statistically significant shrinkage of 0.53 ± 0.28% and 0.77 ± 0.17%, respectively (). For the VPS, the group that experienced the least changes to the dimensions of the samples was the hypochlorite, with a statistically significant shrinkage of 0.36 ± 0.09%. The autoclave group and the control group were the groups where there was greater dimensional change of the samples, with a shrinkage of 0.57 ± 0.11% and 0.41 ± 0.12% ().
Discussion
Dimensional stability of impression materials has been a constant subject of study and researchers have at their disposal various methods for evaluating the dimensional stability [Citation16,Citation35]. This study resorted to ADA specification no. 19 and ISO 4823:2000, since it is an easily reproducible method among investigators, allowing direct comparison between studies with various materials [Citation20]. However, as with any in vitro investigation there are limitations. The accuracy of this method depends upon the operator who is in charge of the measurements and the microscopic readability [Citation6]. Another disadvantage is the fact that the materials tested did not present a clinically relevant shape and the impression is performed without the presence of moisture or saliva. This means that the execution and removal of the impressions, as well as the deformation suffered does not mimic the clinical condition [Citation2,Citation5–7,Citation32]. However, as the objective of the study is to evaluate dimensional changes introducing the variable time, sterilization, and disinfection, it becomes important to reduce the number of associated variables. A third limitation is based on the fact that the measurements are carried out on a flat surface eliminating the possibility to detect dimensional changes in three dimensions [Citation6]. The fact that the two materials have comparable viscosities did not affect the outcome as the results are very similar.
The Michelson method allows a direct measurement of the sample and has a high accuracy and precision of results [Citation36,Citation37]. ISO 4823:2000 requests an accuracy of 0.01 mm for the measuring technique. However, in the present study, the use of a Michelson interferometer allowed an accuracy of 0.00001 mm.
The samples were subjected to two different procedures, all common in dental practice. In the control group, no sterilization or disinfection procedure was performed, and this group was used for comparative purposes and control of the behavior of both materials.
A disinfection protocol by immersion of the samples in a sodium hypochlorite solution at 5.25% for 10 min was employed in the hypochlorite group. This concentration is known to have virucidal, fungicide and bactericide properties and is adequate to allow disinfection [Citation6,Citation31]. We chose an immersion time of 10 min since there are several authors advocating it [Citation6,Citation15,Citation22,Citation23,Citation30,Citation31]. This time is below the maximum immersion period allowed by ADA, which is 30 min [Citation6]. One can question why the ADA does not establish a minimum time period. Adabo et al. [Citation15] state that the disinfection of PE and VPS with a 5.25% hypochlorite solution for 10 min is not only effective in reducing the number of microorganisms present on the material surface, it causes no significant dimensional changes in the impressions. Langenwalter and Tullner, who studied the same materials with similar disinfection protocols, also obtained the same conclusions [Citation23,Citation30]. Kern et al. [Citation22] made use of an immersion of the impressions in a solution of glutaraldehyde and sodium hypochlorite for 10 min and observed that these two disinfection protocols do not cause clinically significant adverse effect on the dimensional stability of the impressions.
In the autoclave group, the samples were subjected to a 40-min sterilization cycle at 134 °C in a steam autoclave as this is considered the most effective method of sterilization. Nonetheless, there are insufficient studies on the effects of this procedure on the stability and reproducibility of the impressions [Citation20]. Nassar et al. studied the dimensional stability of a VPS during 2 weeks of storage and found small dimensional changes that were statistically significant [Citation5].
The results obtained in our study demonstrate a statistically significant distortion of the samples during the 6 months of storage. The ISO 4823:2000 sets a maximum dimensional change of 1,5% for type 0 materials, such as PE and VPS [Citation34]. However, the maximum dimensional changes obtained in this study were 0.90 ± 0.26%, which shows that the dimensional changes over the storage of the materials are not clinically significant.
Some authors claim that disinfection and sterilization have no adverse effect on the dimensional stability of impressions [Citation15,Citation24,Citation30], while others point to possible adverse effects [Citation25,Citation31]. Tullner et al. [Citation30] found that the disinfection or sterilization of impressions did not produce clinically significant changes in their dimensions. Other authors noted, as showed in this study for both materials, that the disinfection of the impressions brings in the long term benefits over the impressions which have not undergone this process [Citation25,Citation31]. In fact, samples subjected to disinfection by immersion in 5.25% sodium hypochlorite solution after 6-month storage showed less dimensional change when compared with the control group and autoclave group.
Thouati et al. [Citation31] observed, after disinfection of the elastomers by immersion in a 5.25% hypochlorite solution for 30 min, a significant dimensional change of the impressions (between −0.008% and 0.29%), and that this dimensional change is smaller when compared to the impressions that did not undergo disinfection by immersion. Although the disinfection in our study was carried out for only 10 min, our results are corroborated by the previous work of Thouati et al. [Citation31]. For the PE hypochlorite group, after 6 months, shows a shrinkage of 0.64% and the control group0.90%; VPS hypochlorite group has a shrinkage of 0.52% and 0.53% in the control group. In fact, the effect of the disinfectant solution was not significant on the dimensional variations of these materials, showing that applying cross-control infection procedures, such as disinfection, is favorable from a clinical viewpoint. Martin and Jedynakiewicz in 2007 stated that Aquasil® Monophase (Dentsply, Caulk) had a very low shrinkage of just 0.05% and ImpregumTM F (3 M ESPE, St Paul, MN) presented a greater dimensional change after immersion in sodium hypochlorite 5.25% [Citation36]. The fact that the PE is a hydrophilic material justifies the further dimensional change that this material undergoes when compared with VPS [Citation36]. This idea is supported by our results, since the PE hypochlorite group suffered a shrinkage of 0.64% and the VPS hypochlorite group a shrinkage of 0.52%. Nassar and Chow [Citation26] suggest that because of the hydrophilic structure of PE this material imbibes a greater amount of disinfectant solution during the disinfecting procedure resulting in a higher dimensional change and a greater shrinkage due to the loss of volatile components over time.
This study did not further elucidate the reason why the dimensional changes in both materials were inferior in the hypochlorite group. According to Sinobad et al chlorine at 5.25% is a highly reactive element and could react with impression materials and adhere on the material [Citation6]. However, Oda speculated that the samples disinfected with hypochlorite at 5.25% may have some disinfectant uptake during the disinfection procedure [27]. This interaction between the hypochlorite and the constituents of the impression material might result in a ‘sealing’, preventing or reducing the dimensional change over time. It seems there are indeed possible beneficial effects of disinfection by immersion in 5.25% NaOCl for 10 min [Citation27]. After this period, there is a chemical stabilization where minor changes in the dimension of the material can occur [Citation6,Citation27].
According to Thota et al. [Citation20] autoclaving is the most effective sterilization procedure for VPS, but for PE this procedure is not recommended. For PE, the disinfection procedure should be the one specified by the manufacturer. However, the present study found that the autoclave group for VPS has a shrinkage over the 6 months of 0.87%, higher than the hypochlorite group, in other words, the largest dimensional change resided in the autoclave group.
This study revealed no statistically significant differences between VPS and PE when compared at T0. In T1 there were only statistically significant changes between the two materials in the control group, while the PE has a higher shrinkage than VPS. These results can be explained by the hydrophilic nature of PE [Citation23,Citation28,Citation29]. Chen et al. [Citation38] concluded that prolonged storage causes dimensional changes in VPS; however, these changes are lower when compared with those which occur in other materials. Nassar studied the dimensional change in two VPS materials EXA’lence 370 Monophase (GC America Inc Alsip, III and ImprintTM 3 Monophase (3 M ESPETM, St Paul, MN) and one PE ImpregumTM PentaTM (3 M ESPETM AG, Seefeld, Germany) after two weeks of storage and concluded that changes in PE are higher than in VPS; however, in both types, these changes are not clinically significant [Citation5]. Walker et al. [Citation39] evaluated the dimensional stability of a PE and a VPS after disinfection after a two-week storage period and concluded that the PE showed significant dimensional changes after storage, while the VPS did not show significant changes in its dimension.
Both materials exhibit dimensional changes when subjected to disinfection and sterilization. Hence, the null hypothesis at the 5% level is rejected. The long-term storage of samples subjected to disinfection with 5.25% hypochlorite or autoclave sterilization can be used in a clinical setting as the dimensional changes are below the maximum permitted by the ISO 4823:2000, since there are no clinically significant changes in the dimension of the samples during the storage period.
More studies on disinfection, sterilization, and storage conditions of impression materials are necessary to ensure greater success of clinical procedures and cross-contamination prevention.
Acknowledgements
The authors would like to thank 3M ESPE Portugal for the materials and the Portuguese Institute of Quality for the use of the facilities.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rubel BS. Impression materials: a comparative review of impression materials most commonly used in restorative dentistry. Dent Clin N Am. 2007;51:629–642.
- Pant R, Juszczyk AS, Clark RKF, et al. Long-term dimensional stability and reproduction of surface detail of four polyvinyl siloxane duplicating materials. J Dent. 2008;36:456–461.
- Kumar RN, Reddy SM, Karthigeyan S, et al. The effect of repeated immersion of gypsum cast in sodium hypochlorite and glutaraldehyde on its physical properties: an in vitro study. J Pharm Bioall Sci. 2012;4:353–357.
- Hamalian TA, Nasr E, Chidiac JJ. Impression materials in fixed prosthodontics: influence of choice on clinical procedure. J Prosthodont. 2011;20:153–160.
- Nassar U, Oko A, Adeeb S, et al. An in vitro study on the dimensional stability of a vinyl polyether silicone impression material over a prolonged storage period. J Prosthet Dent. 2013;109:172–178.
- Sinobad T, Obradović-Djuricić K, Nikolić Z, et al. The effect of disinfectants on dimensional stability of addition and condensation silicone impressions. Vojnosanit Pregl. 2014;71:251–258.
- Gonçalves FS, Popoff DAV, Castro CDL, et al. Dimensional stability of elastomeric impression materials: a critical review of the literature. Eur J Prosthodont Restor Dent. 2011;19:163–166.
- Anusavice KJ, Phillips RW, Shen C, et al. Phillips’ science of dental materials. 12th ed. St. Louis (Mo): Elsevier/Saunders; 2013.
- Donovan TE, Chee WWL. A review of contemporary impression materials and techniques. Dent Clin North Am. 2004;48:vi–470.
- Craig RG, Sun Z. Trends in elastomeric impression materials. Oper Dent. 1994;19:138–145.
- Franco EB, da Cunha LF, Benetti AR. Effect of storage period on the accuracy of elastomeric impressions. J Appl Oral Sci. 2007;15:195–198.
- Grundke K, Michel S, Knispel G, et al. Wettability of silicone and polyether impression materials: characterization by surface tension and contact angle measurements. Physicochem Eng Asp. 2008;317:598–609.
- Surapaneni H, Pallavi Samantha P, Ravi Shankar Y, et al. Polyvinyl siloxanes in dentistry: an overview. Trends Biomater Artif Organs. 2013;27:115–123.
- Silva SM, Salvador MC. Effect of the disinfection technique on the linear dimensional stability of dental impression materials. J Appl Oral Sci. 2004;12:244–249.
- Adabo GL, Zanarotti E, Fonseca RG, et al. Effect of disinfectant agents on dimensional stability of elastomeric impression materials. J Prosthet Dent. 1999;81:621–624.
- Amin WM, Al-Ali MH, Tarawneh SK, et al. Effects of disinfectants on dimensional accuracy and surface quality of impression materials and gypsum casts. J Clin Med Res. 2009;1:81–89.
- Rios MP, Morgano SM, Stein RS, et al. Efects of chemical disinfectant solutions on the stability and accuracy of the dental impression complex. J Prosthet Dent Thes. 1996;76:356–262.
- Vasconcellos FE, Andreiuolo RF, Sabrosa CE, et al. Dimensional stability of casts obtained with polyether and addition silicone after disinfection with sodium hypochlorite and peracetic acid. Rev Bras Odontol. 2012;69:55–60.
- Abdelaziz KM, Hassan AM, Hodges JS. Reproducibility of sterilized rubber impressions. Braz Dent J. 2004;15:209–213.
- Thota KK, Jasthi S, Ravuri R, et al. A comparative evaluation of the dimensional stability of three different elastomeric impression materials after autoclaving – an invitro study. J Clin Diagn Res. 2014;8:48–50.
- Endo T, Finger WJ. Dimensional accuracy of a new polyether impression material. Quintessence Int. 2006;37:47–51.
- Kern M, Rathmer RM, Strub JR. Three-dimensional investigation of the accuracy of impression materials after disinfection. J Prosthet Dent. 1993;70:449–456.
- Langenwalter EM, Aquilino SA, Turner KA. The dimensional stability of elastomeric impression materials following disinfection. J Prosthet Dent. 1990;63:270–276.
- Kronström MH, Johnson GH, Hompesch RW. Accuracy of a new ring-opening metathesis elastomeric dental impression material with spray and immersion disinfection. J Prosthet Dent. 2010;103:23–30.
- Lucas MG, Arioli-Filho JN, Nogueira SS, et al. Effect of incorporation of disinfectant solutions on setting time, linear dimensional stability, and detail reproduction in dental stone casts. J Prosthodont. 2009;18:521–526.
- Nassar U, Chow AK. Surface detail reproduction and effect of disinfectant and long-term storage on the dimensional stability of a novel vinyl polyether silicone impression material. J Prosthodont. 2015;24:494–498.
- Oda Y, Matsumoto T, Sumii T. Evaluation of dimensional stability of elastomeric impression materials during disinfection. Bull Tokyo Dent Coll. 1995;36:1–7.
- Chia WK, Stevens L, Basford KE, et al. Dimensional change of impressions on sterilization. Aust Dent J. 1990;35:23–26.
- Salem N, Combe EC. The effects of chemical sterilisation on the dimensional stability of some elastomeric impression materials. Clin Mater. 1990;6:75–82.
- Tullner JB, Commette JA, Moon PC. Linear dimensional changes in dental impressions after immersion in disinfectant solutions. J Prosthet Dent. 1988;60:725–728.
- Thouati A, Deveaux E, Iost A, et al. Dimensional stability of seven elastomeric impression materials immersed in disinfectants. J Prosthet Dent. 1996;76:8–14.
- Walker MP, Rondeau M, Petrie C, et al. Surface quality and long-term dimensional stability of current elastomeric impression materials after disinfection. J Prosthodontics. 2007;16:343–351.
- Pimentel L, Portugal J, Vasconcelos M, et al. Influence of temperature on the accuracy of an autoclaved addition silicone. Rev Port Estomatol Med Dentária e Cir Maxilofac. 2014;55:43–48.
- International Organization for Standardization. 4823:2000 Dentistry – Elastomeric impression materials. ISO. International Standard ISO; 2000.
- Marković D, Puškar T, Hadžistević M, et al. The dimensional stability of elastomeric dental impression materials. Contemp Mater. 2012;3:105–110.
- Martin N, Martin MV, Jedynakiewicz NM. The dimensional stability of dental impression materials following immersion in disinfecting solutions. Dent Mater. 2007;23:760–768.
- Quick DC, Holtan JR, Ross GK. Use of a scanning laser three-dimensional digitizer to evaluate dimensional accuracy of dental impression materials. J Prosthet Dent. 1992;68:229–235.
- Chen SY, Liang WM, Chen FN. Factors affecting the accuracy of elastometric impression materials. J Dent. 2004;32:603–609.
- Walker MP, Petrie CS, Haj-Ali R, et al. Moisture effect on polyether and polyvinylsiloxane dimensional accuracy and detail reproduction. J Prosthodontics. 2005;14:158–163.

