Abstract
This study was conducted to determine whether novel experimental low-shrinkage dimethacrylate co-monomers could provide low polymerization shrinkage composites without sacrifice to degree of conversion, and mechanical properties of the composites. Experimental composites were prepared by mixing 28.6 wt% of bisphenol-A-glycidyl dimethacrylate based resin matrix (bis-GMA) with various weight-fractions of co-monomers; tricyclo decanedimethanol dacrylate (SR833s) and isobornyl acrylate (IBOA) to 71.4 wt% of particulate-fillers. A composite based on bis-GMA/TEGDMA (triethylene glycol dimethacrylate) was used as a control. Fracture toughness and flexural strength were determined for each experimental material following international standards. Degree of monomer-conversion (DC%) was determined by FTIR spectrometry. The volumetric shrinkage in percent was calculated as a buoyancy change in distilled water by means of the Archimedes’ principle. Polymerization shrinkage-strain and -stress of the specimens were measured using the strain-gage technique and tensilometer, respectively with respect to time. Statistical analysis revealed that control group had the highest double-bond conversion (p < .05) among the experimental resins tested. All of the experimental composite resins had comparable flexural strength, modulus, and fracture toughness (p > .05). Volumetric shrinkage and shrinkage stress decreased with increasing IBOA concentration. Replacing TEGDMA with SR833s and IBOA can decrease the volumetric shrinkage, shrinkage strain, and shrinkage stress of composite resins without affecting the mechanical properties. However, the degree of conversion was also decreased.
Introduction
With advances in dentin adhesives and the evolution of esthetic dentistry in 1990s, composite resins have become more widely used in restorative dentistry [Citation1]. The longevity of composite restorations, as well as the durability of the composite resin material itself as a tooth replacement, is often questioned and multi-factorial. Many believe that the most serious issue with dental composites is the fact that the polymerization reaction is accompanied by a volumetric shrinkage that generates stress within the material. This shrinkage stress may result in interfacial bond failures, microleakage, deformation of the tooth cusps, post-operative sensitivity and in the long-run secondary caries [Citation1,Citation2]. Thus, advanced studies have been undertaken to improve composite resins in order to have a material with low polymerization shrinkage and high strength keeping in mind the requirements of esthetic properties. Many factors affect the shrinkage of composite resins, including resin matrix composition, filler content, and the polymerization method [Citation3]. Attempts have been made to change the type of fillers or filler size and their surface silanization. By changing the polymerization kinetics of resin matrices and degree of monomer conversion, further attempts have been made to influence the resultant shrinkage [Citation4,Citation5]. However, polymerization shrinkage-shrinkage stress when monomer molecules are converted into a polymer network, still remains a challenge. Different measurement techniques were used to follow and to understand this phenomenon, including the mercury dilatometric technique, the bonded-disc technique, strain-gage methods, shrinkage-stress tests and laser interferometry [Citation6]. Though, the comparison between these device set-ups and clinical situations is difficult [Citation2,Citation6].
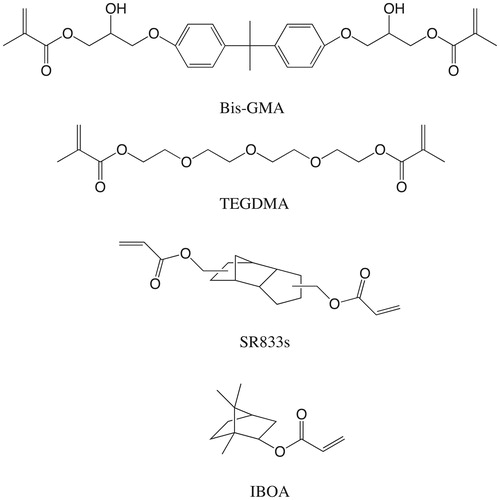
As an important factor affecting the development of shrinkage stress in dental composites, volumetric shrinkage combined with polymerization of resin matrices should be reduced. Triethylene glycol dimethacrylate (TEGDMA) was introduced into resin matrices as a diluent to overcome the drawbacks of 2,2-bis[p-(2′-hydroxy-3′-methacryloxypropoxy)phenyl] propane (bis-GMA), such as high viscosity and low double-bond conversion under ambient polymerization conditions [Citation7–10]. However, compared with bis-GMA (shrinkage as 5.2%), TEGDMA (shrinkage as 12.5%) has much higher volumetric shrinkage, thus using diluents with lower volumetric shrinkage is an effective way to reduce overall shrinkage of resin matrices [Citation11]. Tricyclo (5.2.1.0) decanedimethanol dacrylate (SR833s) and isobornyl acrylate (IBOA) are two kinds of low-viscous acrylate monomers reported as substitutes for TEGDMA in resin matrices with the aim of reducing volumetric shrinkage, but their influences on polymerization shrinkage behavior of dental composite resins has not yet been investigated [Citation12,Citation13].
Based on this knowledge, the purpose of the present study was to evaluate the polymerization shrinkage behavior (volumetric shrinkage, shrinkage strain and shrinkage stress) and physical properties (degree of conversion, flexural strength and modulus and fracture toughness) of novel experimental low-shrinkage dental composite resin. The null hypotheses were: (i) experimental low-shrinkage composites will promote a similar degree of conversion and lower shrinkage behavior compared to conventional composite resin; and (ii) experimental low-shrinkage composites will achieve similar mechanical properties compared to convention composite resin.
Materials and methods
Materials
bis-GMA and TEGDMA were purchased from Esstech Inc. (Essington, PA). IBOA, camphoroquinone (CQ), N,N′-dimethylaminoethyl methacrylate (DMAEMA) were purchased from Sigma-Aldrich (St. Louis, MO). SR833s was obtained from Sartomer Company Inc. (Exton, PA). All of reagents were used without purification. Silaned BaAlSiO2 filler particles (Ø 0.7 µm) were received from Schott (UltraFine UF 0.7 µm; Schott, Landshut, Germany). The chemical structures of all used monomers are presented in .
Preparation of experimental dental composites
Resin matrices of dental composites were prepared according to the formulations shown in . All compounds were weighed and mixed under magnetic stirring. Experimental dental composites were prepared by mixing each resin matrix with fillers in a high-speed mixing machine (SpeedMixer, DAC150 FVZ-K; Hauschild, Hamm, Germany) with a speed of 1900 rpm. The mass ratio between resins matrix and fillers was 2/5 (wt/wt).
Table 1. Composition of resin matrix for each experimental composite.
Double-bond conversion
Double-bond conversion (DC%) during and after the photoinitiation of polymerization was monitored by Fourier-transform infrared spectroscopy (FTIR) (Spectrum One, Perkin-Elmer, Beaconsfield Bucks, UK) with an attenuated total reflectance (ATR) accessory. Composites were analyzed in a mold that was 1.5 mm thick and 4.5 mm in diameter. First, the spectrum of the unpolymerized sample was placed in the mold and measured. Then, the sample was irradiated through an upper glass slide for 40 s with a visible light-curing unit (Elipar™ S10, 3M ESPE, Landsberg am Lech, Germany) producing an average irradiance of 1800 mW/cm2 (Marc Resin Calibrator, BlueLight Analytics Inc., Halifax, Canada). The sample was scanned for its FTIR spectrum after being irradiated. The DC was calculated from the aliphatic C = C peak at 1636 cm−1 and normalized against the carbonyl C = O peak at 1720 cm−1 according to the formula:
where AC = C and AC = O were the absorbance peak area of methacrylate C = C at 1636 cm−1 and carbonyl at 1720 cm−1, respectively (AC = C/AC = O)0 and (AC = C/AC = O)t represented the normalized absorbency of the functional group at the radiation time of 0 and t, respectively; DC is the conversion of methacrylate C = C as a function of radiation time. For each composite, five trials were performed.
Mechanical tests
Three-point bending test specimens (2 × 2×25 mm3) were made from each tested composite. Bar-shaped specimens were made in a half-split stainless steel molds between transparent Mylar sheets. Polymerization of the materials was done using a hand light-curing unit (Elipar S10, 3 M ESPE, St. Paul, MN) for 20 s in five separate overlapping portions from both sides of the metal mold. The wavelength of the light was between 430 and 480 nm and light intensity was 1600 mW/cm2. The specimens from each material (n = 8) were stored dry (for 1 day) at 37 °C before testing. The three-point bending test was conducted according to the ISO 4049 (test span: 20 mm, cross-head speed: 1 mm/min, indenter: 2 mm diameter). All specimens were loaded into a material testing machine (model LRX, Lloyd Instrument Ltd., Fareham, UK) and the load-deflection curves were recorded with a PC software (Nexygen 4.0, Lloyd Instruments Ltd.).
Flexural strength (o’f) and flexural modulus (Ef) were calculated from the following formula (ISO 1992):
where Fm is the applied load (N) at the highest point of a load-deflection curve, L is the span length (20 mm), b is the width of test specimens and h is the thickness of test specimens. S is the stiffness (N/m). S = F/d and d is the deflection corresponding to load F at a point in the straight-line portion of the trace.
Single-edge-notched-beam specimens (2.5 × 5×25 mm3) according to adapted ISO 20795-2 standard method (ASTM 2005) were prepared to determine the fracture toughness. A custom-made stainless steel split mold was used, which enabled the specimen’s removal without force. An accurately designed slot was fabricated centrally in the mold extending until its mid-height, which enabled the central location of the notch and optimization of the crack length (x) to be half of the specimens’ height. The restorative material was inserted into the mold placed over a Mylar-strip-covered glass slide in one increment. Before polymerization, a sharp and centrally located crack was produced by inserting a straight-edged steel blade into the prefabricated slot. Polymerization of the composite was carried out for 20 s in five separate overlapping portions. The upper side of the mold was covered with Mylar strip and glass slide from both sides of the blade, before being exposed to the polymerization light. Upon the removal from the mold, each specimen was polymerized also on the opposite side. The specimens from each group (n = 8) were stored dry at 37 °C for 24 h before testing. The specimens were tested in three-point bending mode, in a universal material testing machine at a crosshead speed of 1.0 mm/min.
The fracture toughness was calculated using the equation:
where f(x) = 3/2 × 1/2 [1.99 − × (1 − x) (2.15–3.93x + 2.7 × 2)]/2(1 + 2x) (1 − x)3/2 and 0 < x < 1 with x = a/W. Here, P is the maximum load in kilonewtons (kN), L is the span length (20 cm), B is the specimen thickness in centimeters (cm), W is the specimen width (depth) in cm, x is a geometrical function dependent on a/W and a is the crack length in cm.
Shrinkage-stress measurement
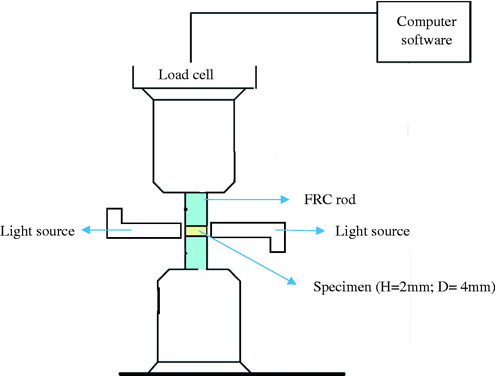
Glass fiber reinforced composite rods with 4 mm diameter and 4 cm length had one of their flat surfaces ground with 180 grit silicon carbide sand paper. Two FRC rods were attached tightly to a universal testing machine (model LRX, Lloyd Instruments Ltd.) and tested material was applied between the FRC rod surfaces (). The height of the specimen was set at 2 mm. Two light units (Elipar S10, 3M ESPE, St. Paul, MN) were used simultaneously for 20 s with the tips in close contact with the material specimen from both sides. Contraction forces were monitored for 5 min at room temperature (22 °C). Shrinkage stress was calculated by dividing the shrinkage force by the cross-section area of the FRC rod. The maximum shrinkage-stress value was taken from the plateau at the end of shrinkage stress/time curve. Five specimens were tested for each experimental material.
Shrinkage-strain measurement
The polymerization shrinkage strain was monitored using the strain gage method. This method was previously described by Sakaguchi [Citation14]. The uncured materials were placed in a silicon mold (10 × 10 × 1.5 mm3) on top of one uniaxial strain gage. The strain gage (KFG-2N-120, Kyowa Ltd., gage length 2 mm) were used to measure shrinkage strains at room temperature (22 °C). The materials were placed on the polyimide backing of the strain gage on the opposite side of the electrical resistance foil without any adhesive. The adhesion between the resin paste and the strain gage was previously shown to be sufficient to transfer all the contraction strain from the resin to the gage [Citation14]. Polymerization shrinkage data were acquired from one strain gage using a strain measurement module (PCD-300A, Kyowa Ltd., Fukuokapref, Japan), which had been initially balanced at zero. The sampling rate of the module was 10 Hz. Data collection started 10 s before the start of polymerization and continued for 400 s. Both upper and lower surfaces of the specimens (n = 5) were covered with a separating sheet and a glass plate and irradiated for 20 s with a hand-held light-curing unit (Elipar S10, 3M ESPE, St. Paul, MN). The light curing tip was maintained at a 2 mm distance above the glass slide with the use of a reference plate.
Volume shrinkage
The specimens’ densities (n = 3) were measured to determine volume shrinkage according to Archimedes’ principle with a commercial density determination kit of the analytical balance (XS105; Mettler Toledo, Greifensee, Switzerland). The mass of the specimen was weighed in air and water, and density was calculated according to the equation:
where D is the density of the sample, M1 is the mass of the sample in air, M2 is the mass of the sample in water, and Dw is the density of water at the measured temperature. For each composite, six trials were performed respectively to calculate the densities of polymerized and unpolymerized samples. The volume shrinkage (VS) was expressed in % and calculated from the densities according to the equation:
where Du is the density of the unpolymerized sample and Dc is the density of the polymerized sample.
Statistical analysis
The data were statistically analyzed with SPSS version 23 (SPSS, IBM Corp., Armonk, NY) using analysis of variance (ANOVA) at the p < .05 significance level followed by a Tukey’s HSD post hoc test to determine the differences between the groups.
Results
The values of DC, FS, FM, and FT of the experimental dental composite resins were listed in . The results showed that the control group (bis-GMA/TEGDMA) had the highest DC (p < .05) among all dental composite resins. The EC-1, EC-2, and EC-3 had comparable DC (p > .05). The DC of EC-4 was higher than those of EC-1 and EC-2 (p < .05), but similar to DC of EC-3 (p > .05). All of experimental dental composite resins had comparable FS, FM, and FT (p > .05).
Table 2. Degree of conversion and mechanical properties mean values (±standard deviation) of investigated composites.
The results of VS were presented in . As shown, the control group had the highest VS (p < .05) among all experimental dental composite resins. The VS of EC-4 was same as VS of EC-2 and EC-3 (p > .05), but lower than that of EC-1 (p < .05).
Table 3. Volumetric shrinkage, shrinkage strain, and shrinkage stress of investigated composites.
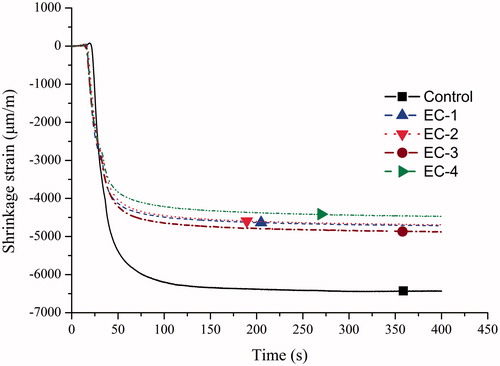
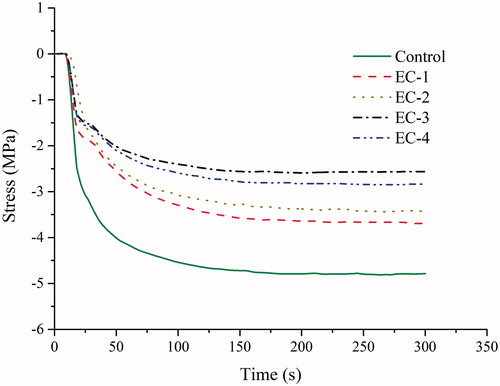
and displayed the curves of shrinkage strain versus time and shrinkage stress versus time, respectively, and final shrinkage strain and stress were summarized in . For all groups, shrinkage strain and stress developed rapidly after the start of light curing and then increased at a slower rate up to the fixed measure time. The control group had higher shrinkage strain and stress (p < .05) than the other dental composite resins. While the other composites had comparable shrinkage strain (p > .05). Shrinkage stress of EC-1 was the same as that of EC-2 (p > .05) but higher than those of EC-3 and EC-4 (p < .05). The EC-2, EC-3, and EC-4 exhibited the comparable shrinkage-stress values ().
Discussion
As an important parameter, monomeric composition determines all the performances of dental composite resins. In this research, reactive diluent TEGDMA in the control group was replaced by SR833s and IBOA, and this should be the main reason for the differences between each experimental dental composite resin.
As expected, the polymerization shrinkage of the low-shrinkage experimental composite resins was reduced in comparison to the control composite resin (). All methacrylate based resins shrink to some extent, and contraction can be reduced by using monomers with a high molecular weight or low reactive group concentration [Citation15,Citation16]. Compared with the structure of TEGDMA, there existed rigid aliphatic ring in SR833s, which could induce earlier time to vitrification and trap more unreacted double bonds in the polymeric network, leading to lower DC [Citation17]. Though, when SR833s was replaced by IBOA, cross-linking density of polymeric network was reduced because of the mono-functional structure of IBOA, which could lead to the delay of vitrification time and higher DC [Citation18]. That was why EC-4 with 15 wt.% of IBOA had higher DC than EC-1 without IBOA and EC-2 with 5 wt.% of IBOA. This result was consistent with previous research that, when used as a replacement for TEGDMA, SR833s could reduce DC of dental resin while IBOA could maintain it [Citation12,Citation13,Citation19]. However, the first-tested hypothesis that low-shrinkage composite resins would promote a similar degree of conversion and lower shrinkage behavior compared to the conventional composite resin formulation was partially rejected.
The magnitude of polymerization shrinkage stress has been determined to be dependent on the extent of the reaction, volumetric shrinkage, the stiffness of the material and its ability to flow [Citation11,Citation20]. Higher DC and cross-linking density of composites may result in a high degree of stiffness, which ultimately causes high shrinkage stress [Citation21]. However, all of the composite resins had comparable modulus in this study, thus the highest shrinkage stress of the control composite resin (bis-GMA/TEGDMA-based) should be due to its highest volumetric shrinkage and DC. It also could be observed that the control composite resin behaved differently in the shrinkage-stress curve from the other experimental low-shrinkage composite resins (). When the light curing lamp was switched, a short time period of stable curve was observed for SR833s and IBOA containing composite resins. This phenomenon might be attributed to the thermal expansion of SR833s and IBOA resins (heat generated from curing lamp and exothermic reaction) which compensate for the shrinkage stress for a few seconds before they cooled down to room temperature. After this period a very slow increase of stress was recorded.
The cross-linking density and the nature of monomers are two important factors for mechanical properties of dental composite resins [Citation22]. Though all of SR833s and IBOA containing dental composite resins had lower DC than the control group, which meant that the control group had the highest cross-linking density among all of the experimental dental composite resins, there were no significant differences in mechanical properties like FS, FM, and FT between each composite (). This should be attributed to the rigid aliphatic ring in SR833s and IBOA, which could enhance the polymeric network and offset the negative effect on mechanical properties brought out by lower cross-linking density. However, IBOA could not be used to replace TEGDMA alone, for the mechanical properties of dental resin would be reduced significantly by lower cross-linking density induced by the monofunctional structure of IBOA [Citation12]. Thus, the second hypothesis that experimental low-shrinkage composites would promote similar mechanical properties compared to convention composite resin was accepted.
Ferracane and Hilton [Citation2] stated in their review article that most of the potential clinical manifestations (microleakage and cusp deflection) of shrinkage stress can be negated by the presence of excellent adhesion to the tooth structure, thus providing a resistant seal. Hence, further in vitro studies should be evaluated if the different modified low-shrinkage composite resins really reduce the microleakage and cusp deflection of restored teeth.
There are still unclear issues that need to be known regarding the use of these experimental low-shrinkage dimethacrylate co-monomers, like the water sorption, the biocompatibility and the viscosity. Therefore, further research is needed and an assessment of optimizing the formulation of this novel experimental low-shrinkage co-monomers is now in progress.
Conclusion
Within the limitations of this study, replacing TEGDMA with SR833s and IBOA can decrease the volumetric shrinkage, shrinkage strain, and shrinkage stress of dental composite resins without affecting the mechanical properties, although degree of conversion was also decreased.
Acknowledgements
This study belongs to the research activity of BioCity Turku Biomaterials Research Program (www.biomaterials.utu.fi) and it was supported by Stick Tech Ltd.–Member of the GC Group.
Disclosure statement
Author P.V. consults for Stick Tech Ltd., and is a –member of the GC Group in R&D and training.
References
- Ferracane JL. Resin composite-state of the art. Dent Mater. 2010;27:29–38.
- Ferracane JL, Hilton TJ. Polymerization stress-is it clinically meaningful? Dent Mater. 2016;32:1–10.
- Garoushi S, Vallittu PK, Watts DC, et al. Polymerization shrinkage of experimental short glass fiber reinforced composite with semi-inter penetrating polymer network matrix. Dent Mater. 2008;24:211–215.
- Atai M, Watts DC. A new kinetic model for the polymerization shrinkage-strain of dental composites and resin-monomers. Dent Mater. 2006;22:785–791.
- Watts DC. Reaction kinetics and mechanics in photo-polymerised networks. Dent Mater. 2005;21:27–35.
- Kaisarly D, Gezawi ME. Polymerization shrinkage assessment of dental resin composites: a literature review. Odontology. 2016;104:257–270.
- Liu F, Liao L, He J, et al. Evaluation of new tri-methacrylates as a reactive diluent in root canal sealant. J Biomater Sci Polym Ed. 2011;22:1379–1391.
- Moszner N, Salz U. New developments of polymeric dental composites. Prog Polym Sci. 2001;26:535–576.
- Peutzfeldt A. Resin composites in dentistry: the monomer systems. Eur J Oral Sci. 1997;105:97–116.
- Khatri CA, Stansbury JW, Schultheisz CRS, et al. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent Mater. 2003;19:584–588.
- Braga RR, Ballester RY, Ferracane JL. Factors involved in the development of polymerization shrinkage stress in resin-composites: a systematic review. Dent Mater. 2005;21:962–970.
- He J, Liu F, Luo Y, et al. Properties of 2,2-Bis[p-(2’-hydroxy-3’ methacryloxypropoxy)phenyl]propane/Isobornyl (Meth)acrylate compolymers. J Appl Polym Sci. 2012;126:1527–1531.
- Ding Y, Li B, Wang M, et al. Bis-GMA free dental materials based on UDMA/SR833s dental resin system. Adv Polym Technol. 2016;35:21564.
- Sakaguchi RL, Sasik CT, Bunczak MA, et al. Strain gauge method for measuring polymerization contraction of composite restoratives. J Dent. 1991;19:312–316.
- Yap AU, Soh MS. Post-gel polymerization contraction of “low shrinkage” composite restoratives. Oper Dent. 2004;29:182–187.
- de Oliveira DC, Rovaris K, Hass V, et al. Effect of low shrinkage monomers on physicochemical properties of dental resin composites. Braz Dent J. 2015;26:272–276.
- He J, Liao L, Liu F, et al. Synthesis and characterization of a new dimethacrylate monomer based on 5,5’-bis(4-hydroxylphenyl)-hexahydro-4,7-methanoindan for root canal sealer application. J Mater Sci. 2010;21:1135–1142.
- Lu H, Stansbury JW, Nie J, et al. Development of highly reactive mono-(meth)acrylates as reactive diluents for dimethacrylate-based dental resin systems. Biomaterials. 2005;26:1329–1336.
- Huang Q, He J, Lin Z, et al. Physical and chemical properties of an antimicrobial Bis-GMA free dental resin with quaternary ammonium dimethacrylate monomer. J Mech Behav Biomed. 2016;56:68–76.
- Schneider LF, Cavalcante LM, Silikas N. Shrinkage stresses generated during resin-composite applications: a review. J Dent Biomech. 2010;2010:pii:131630.
- Al Sunbul H, Silikas N, Watts DC. Polymerization shrinkage kinetics and shrinkage-stress in dental resin-composites. Dent Mater. 2016;32:998–1006.
- Asmussen E, Peutzfeldt A. Influence of UEDMA, Bis-GMA and TEGDMA on selected mechanical properties of experimental resin composites. Dent Mater. 1998;14:51–56.