Abstract
Fibr-reinforced composites (FRC) have been used successfully for decades in many fields of science and engineering applications. Benefits of FRCs relate to physical properties of FRCs and versatile production methods, which can be utilized. Conventional hand lamination of prefabricated FRC prepregs is utilized still most commonly in fabrication of dental FRC devices but CAD-CAM systems are to be come for use in certain production steps of dental constructions and medical FRC implants. Although metals, ceramics and particulate filler resin composites have successfully been used as dental and medical biomaterials for decades, devices made out of these materials do not meet all clinical requirements. Only little attention has been paid to FRCs as dental materials and majority of the research in dental field has been focusing on particulate filler resin composites and in medical biomaterial research to biodegradable polymers. This is paradoxical because FRCs can potentially resolve many of the problems related to traditional isotropic dental and medical materials. This overview reviews the rationale and status of using biostable glass FRC in applications from restorative and prosthetic dentistry to cranial surgery. The overview highlights also the critical material based factors and clinical requirement for the succesfull use of FRCs in dental reconstructions.
Introduction
This overview reviews fundamental properties of FRC materials which explain their suitability for dental and medical biomaterials. FRC materials is a group of materials which have been first time tested in 1960s but more extensively developed and clinically approved for dental use during the last 30 years and for the medical implant use during the 15 years [Citation1–6]. Principles behind the development of FRC materials are in resolving clinical problems of bulk metals, ceramics and polymers which are time consuming ex vivo fabrication steps of ceramics, metal ion and nanoparticle release from metals and shortcomings which are related to medical imaging and radiation therapy, and lack of toughness and strength for load-bearing dental restorations and surgical implants. Development of new biomaterials toward clinical use has to follow regulations which are covering medical devices and biomaterials in Europe and worldwide. Risks, which relate to the newly developed biomaterials can be controlled by selecting the first applications to be short-term of use or the device to be removable in nature as was made in developing the FRCs and FRC based treatments. Delay in getting FRC for clinical use was due to problems in combining resins systems with reinforcing fibers, in diffuculties in handling the FRC technically and in rebuttal of accepting new type of materials by clinical dental profession and dental laboratory technicians. However, development of the FRC resin systems and understanding of designing principles behind of constructing devices, and the clinical experience, has lead to the use of FRCs in variety of disciplines and applications: in removable prosthodontics [Citation7–11], fixed prosthodontics [Citation12–40] restorative dentistry [Citation41–54], periodontology [Citation54–56], root canal systems [Citation57–67], orthodontics [Citation68,Citation69], and in repairs of fixed prostheses [Citation70,Citation71]. Critical evaluation of the available FRC materials and correct patient selection is of importance for successful use of the material.
Although there are several proven dental materials and treatment options based on conventional dental materials, a large number of the partially edentulous patients are not treated by fixed dental prostheses to replace their missing teeth or to repair their damaged biting function. This is often due to high cost of the state-of-the-art type of treatments by fixed prostheses and due to irreversible damage by the treatment when creating space for metal and ceramic crowns by cutting enamel and dentin of abutment teeth. An ideal material for dental restorations should be moldable in situ, it should form durable adhesion to the underlaying tooth substrate and it should provide high strength and high toughness after being processed. FRC fullfis these requirements from the material science perspective. FRC is a material combination of polymer matrix and reinforcing fibers. Fibers of the composite are the reinforcing phases in the system when the load is applied to the composite. Load is transferred to the fibers and the material becomes strong and tough. The reinforcing fibers can be continuous unidirectional (rovings), continuous bidirectional (weaves), continuous random oriented (mat) or discontinuous oriented of randon fibers.
FRC can be isotropic, orthotropic or anisotropic which means that material properties and dependent of the direction of the fibers: mechanical, optical, curing shrinkage and thermal properties of the FRC are dependent on the fiber quantity and orientation [Citation72–82]. A high quality glass FRC material with continuous unidirectional glass fiber quantity of 65 vol% in well polymerized dimethacrylate thermoset polymer matrix provide high flexural strength of up to 1250 MPa [Citation72]. No significant reduction of flexural strength and modulus of elasticity by hydrolytic effect of water even in long term water storage of up to 10 years of glass FRC occurs which demonstrates the hydrolytic stability of good quality glass fibers and their silane coupling agent mediated adhesion with the polymer matrix [Citation74,Citation75].
Clinical use of dental FRCs: removable dentures
The first clinical applications for using reinforcing fibers was made with removable dentures which are known to be prone for denture base fractures due to fatigue [Citation83–86]. The problem of denture base fractures has become even higher by the increased use of implant supported overdentures. Glass fibers were selected as the most suitable fibers due to their transluency and possibility to achieve chemical bonding between the fiber and polymer matrix with silane coupling agents [Citation87–89].
The fiber reinforcements in denture bases are divided into two categories. Ladizesky and coworkers reported a method where fibers were distributed through entire denture base [Citation7–11]. This approach is called total fiber reinforcement (TFR). The approach by Vallittu is based on the concept that only the weakest part of the denture base (location of fracture initiation) is reinforced by precisely aligned and positioned fiber reinforcement. This is called as partial fiber reinforcement (PFR) [Citation90]. Clinical studies have been performed with FRC reinforced removable dentures, which suggested that PFR offers an effective and techically easy method to eliminate fractures in denture base [Citation4,Citation5].
Clinical use of dental FRCs: fixed dental prostheses
Today it is known that FRCs can be used to produce definitive fixed dental prostheses (FDPs) although soon after introduction of FRC FDPs in 1990 s this was questioned. FDPs made of FRC are classified as surface retained FDPs, inlay/onlay retained FDPs, full coverage crown retained FDPs and hybrid FDPs [Citation91]. FRC FDPs can be made directly or indirectly. In the FRC FDPs, the framework between the abutments is made of continuous unidirectional fibers. Several laboratory and clinical studies emphasize the effect of correct fiber direction, fiber quantity and interfacial adhesion of veneering resin composites to the FRC framework on the strength of the FDP construction [Citation36,Citation92–97].
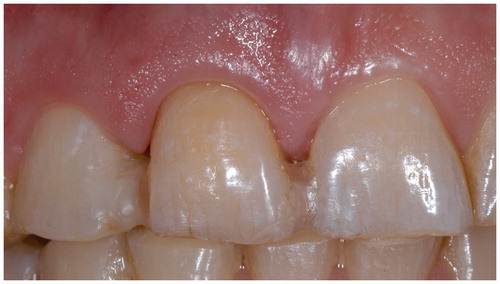
Surface retained FRC FDPs are typically used in anterior region of the dental arch. Inlay/onlay retained FDPs are made by combining the cavities of the abutments by continuous unidirectional fibers and they are preferred in the premolar and molar region. In the premolar and especially in molar region the requirement for the FRC FPD is adequate vertical space for connectors and inlays. In the connectors, four millimeters of vertical space is needed and in the inlays (onlays, crowns) minimum of two millimeters of occlusal space is required for the FRC and overlaying veneering resin composite with a thickness of 1.5 mm [Citation38]. Full coverage crown retained FPDs are made by layering woven FRC and veneering resin composite on prepared abutments. Abutments are connected with continuous unidirectional fibers and by having an additional piece FRC to support cusps of the pontics to eliminate the delamination of the veneer, which is one of the most common type of failure of FRC FPDs [Citation98,Citation99]. Other alternatives to reinforce the pontics are based on using high volume FRC framework for FDP [Citation98,Citation99]. Attempts to use prefabricated pontics made of ceramic materials and using resin based denture teeth have been made. Natural tooth crown can also be used as a pontic for FRC FPD (). It was shown that by using glass ceramics and acid etching and silane priming techniques mechanically stable and reliable pontics were obtained if the occlusal thickness of the pontic material was high enough (4 mm) [Citation100–104]. On the other hand, polymer denture teeth provided reliable pontic system even with 2.5 mm occlusal thickness of the denture tooth [Citation100]. Use of full coverage crowns as retaining elements of FDPs does not allow treatment to be according to the principles of minimal invasiveness like hybrid or inlay retained FDPs, but can offer a lower cost FDP alternative [Citation32]. FRCs can also be used a reinforcements of provisional FDPs during fabrication of conventional FDPs [Citation93].
Figure 1. Use of natural tooth as pontic of FRC FPD. Severely periodontally damaged tooth (A) needs to be extracted (B) and replaced by minimally invasive FPD immediately after extraction.
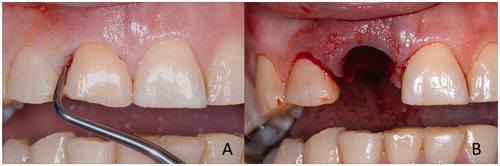
Figure 2. Extracted tooth (A) is cut and veneered from the cutting surface with resin composite (B) to make a pontic (C) for being attached to the adjacent teeth with continuous unidirectional glass fibers (C).
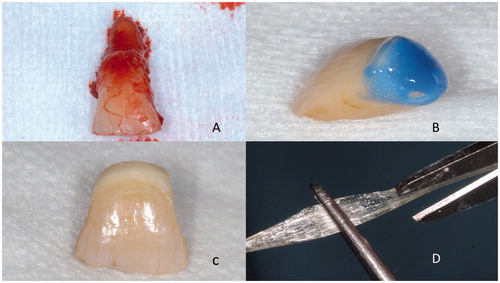
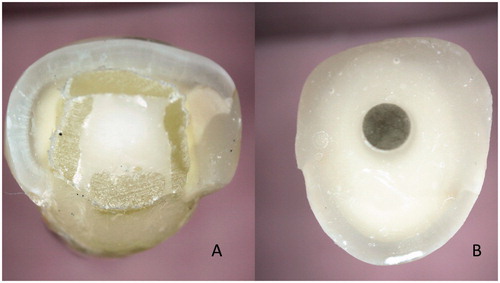
Figure 4. Cross sections of teeth with individually formed FRC post (A) and prefabricated FRC post (B). In the individually formed FRC post system the reinforcing fibers are located closer to the highest stress are of tooth, i.e. surface of the root and the fibers provides better support for the crown than the prefabricated post.
Clinical use of dental FRCs: root canal posts
Endodontically treated tooth with loss of dentin and enamel may need additional support to anchor the restoration. The very first reported fiber composite root canal posts were used in Japan in 1600 century. The posts of that time were made of wood, which is a composite of cellulose fibers and lignin polymer matrix. After starting to use silver posts for retaining crowns in 1800 s, the material of silver was replaced soon by dental gold alloys, which became material of standard for over hundred years of time. Metals posts are structurally and due to material properties rigid constructions, which effectively transfer occlusal loads to the fragile dentin of the root. Repeated stresses cause fatigue of dentin and can cause vertical fracture of the root. By adding so-called extraradicular metal ferrule of width of 1.5 to 2.0 to the crown, the root fractures can to large extent be eliminated. However, the present era of nonmetallic crowns of glass ceramics and resin composites do not have metal ferrule and thus, the root fracture elimination have to be done intraradicularly. So-called modulus compensation is method to lower the magnitude of local stress and prevalence of root fractures in root dentin [Citation32]. The modulus compensation is achieved by selection of post material and post design, which match to the modulus of elasticity of root. Glass FRCs fulfills the requirement of isoelasticity with dentin. The use of FRC in root-canal posts to anchore cores and crowns has rapidly increased although the use of post systems have decreased in general along the development of adhesive techniques and materials, e.g. by introduction of so-called endocrown systems [Citation58–60,Citation65,Citation66]. FRC can be used in root canal as prefabricated solid posts and individually formed posts, the latter representing the most optimal post design () [Citation64,Citation65].
Figure 5. Light microscopic image of discontionuos glass FRC which is used in bilayered direct resin composite restorations.
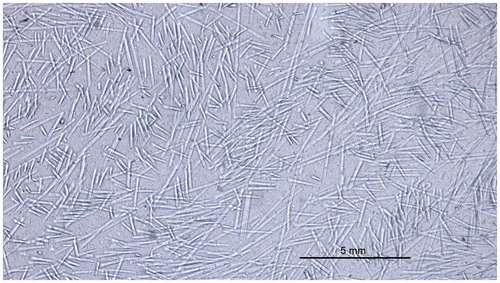
The prefabricated FRC posts are made of reinforcing fibers (carbon/graphite, glass, quartz) and finally polymerized resin matrix between the fibers which form a solid post of a predetermined diameter. Individually formed posts are made of non-polymerized fiber-resin prepregs, consisting of glass fibers and light-curing resin matrix. The rationale of the individually formed FRC post is to fill the entire space of the root canal by FRC material [Citation64,Citation68]. The increased fiber quantity, especially in the coronal part of the root canal increases load-bearing capacity of the system. Biomechanical behavior of restored tooth can also better be simulated because the fibers are located closer to the dentin walls, where the highest stresses exist. FRC close to dentin walls inside the root canal functions as ‘an intraradicular ferrule’. A tooth restored with individually formed root canal posts system withstands cyclic loading of high magnitude for a long period of time without catastrophic failure or marginal breakdown of the crown, which can predispose to the secondary caries. For transferring the occlusal loads from crown to the individually formed FRC post, dentin and periodontium, good bonding between the luting cements, core build-up composites, post and dentin are essential. Adequate bonding of resin composite luting cements and core build-up resin composites to the post can clinically be achieved by using FRC post system where the polymer matrix is composed of interpenetrating polymer network (IPN) resin system which allows monomers of the cement to dissolve the surface of the post [Citation58,Citation105,Citation106]. Cross-linked polymer matrix of all present prefabricated FRC posts does not enable bonding of luting cements or core build-up resin composites to the post and therefore additional mechanical retention of posts and long posts should be used.
Clinical use of dental FRCs: filling resin composites
Although amalgam has shown its many benefits as dental restorative material its use is ending due to environmental reasons. Treatment of damaged tooth structure involves direct resin composite restorations on the population level allowing high cost-effect ratio for the treatment outcome. Particulate filler resin composites have fulfilled direct application requirements in terms of material cost but often failed in terms of longevity of restorations made by general practitioners. One reason for the limited longevity of restorations is low mechanical strength of the particulate filler resin composite as material and inadequately adjusted occlusion, which can cause high local stress concentrations and damage the restoration. Resin composite restorations, like ceramic restorations, do not become adjusted to the occlusion like amalgam restorations did during long lasting setting reaction. Adjustment of occlusion of the resin composite and ceramic restorations must be made by the dentist with high precision.
Utilization of reinforcing fibers in filling composites to toughen the material has been tested for years but not until recently, the reinforcing effect by fibers has been proved [Citation41–54,Citation106]. Reasons for the poor success of previous FRC filling materials have been of selecting of too short discontinuous fibers, which were not even in theory able to increase strength and toughness of the resin composite. The current concept of using FRC in fillings is based on the bilayered composite system in which FRC base is made of discontinous fibers with length of the fibers exceeding the critical fiber length in the dimethacrylate polymer matrix (). Fibers in the FRC increase toughness and other physical properties of the material compared to regular filling composites [Citation44–54]. Although it is known that protein and microbal adhesion of glass FRC does not considerably differ from that of particulate filler resin composites, the occlusal surface of the FRC is covered with more polishable and wear resistant particulate filler resin composite. The function of the FRC base for filling composites is to provide a crack propagation prevention layer for the restoration. The bilayered resin composite structure is considered as a biomimetic restoration system by mimicking the fibrous structure of dentin-enamel complex [Citation106].
Facial prostheses and FRC
In the development of facial prostheses many different materials have been tested. Currently silicone elastomers are the most commonly used material combined with base material of polymethyl methacrylate. Polymethylmethacrylate base of the facial prostheses if heavy and rigid, and edges of the prostheses do not always lie tightly against the skin during facial experssions and jaw movements. To overcome these problems skeleton of glass FRC was introduced [Citation107–111]. Veneering silicone is bonded to the glass FRC skeleton by help of priming compounds [Citation107] and during the use of the prostheses the edges of the prostheses and slightly compressing the skin keeping it in tight contact with the soft tissues. Compression of the skin by the FRC skeleton has not been shown to affect the microcirculation of the facial skin [Citation112].
Surgical applications for FRC
Durable and tough FRC materials have proven their suitability to surgical applications of implantology. The use of FRC in combination of bioactive modifiers like bioactive glass eliminates several shortcomings of bulk material made implants of metals, ceramics and polymers [Citation113,Citation114]. To improve osteoconductivity and osteoinductivity of the FRC material, particles of bioactive glass (BG) have been added to the surface or inner space of FRC implants [Citation115]. Because radiopacity of glass FRC corresponds to that of cortical bone, there are no artifacts in the diagnostic images but the implant can be seen in the x-rays, CTs and MRIs. Radiation therapy can also be given in the presence of FRC implant. The need for skull reconstructions is increasing mainly due to an increase in decompressive craniectomies, a life-saving maneuver to relieve intracranial pressure resulting from swelling of the brain due to e.g. trauma or cerebrovascular accidents [Citation114].
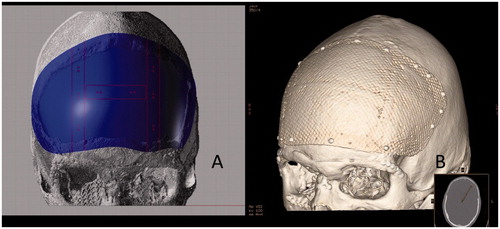
Presently, the most commonly used fibers in medical FRC are made of glass of specific composition but carbon/graphite fibers have also been tested as spinal fusion cages. Glass fibers used in the implants differ from those most commonly used in dental reconstructions. Surgically used glass fibers are referred as S-glass and they are basically free of leaching ions in physiologically moist environment like in living tissues with presence of extracellular liquid. Use of carbon/graphite fibers has been limited due to risk of release of micro and nanometer scale carbon wear debris to tissues. Glass fibers of diameter 15–17 micrometers are used in implants as continuous fibers which have been woven to textile form before impregnating and coupling with resin, and therefore release of wear debris has not found to be a problem. In the presently used designs of FRC implants, both woven textile form of fibers and unidirectional continuous fibers are used in the implant construction. The role of continuous unidirectional fibers is to connect the outer and inner surface laminates together for providing high strength to the implant [Citation108]. Special features of the FRC cranial implant construction are mesh-like surface laminates and presence of free space between the outer and inner laminates, which is loaded with particles of bioactive glass () [Citation114].
Figure 7. Computer aided design of patient specific FRC-BG implant for reconstructing the defect area (A) and computer tomography reconstruction (B) of the FRC-BG implant after cranioplasty operation (Courtesy by Professor Willy Serlo, Oulu University Hospital, Finland).
Long-term durability of the cranioplasty implant is important because according to the present best knowledge, the cranial defects need years of time to be closed by new forming bone even the presence of osteoinductive implant materials [Citation114]. This is the reason why any of the biodegradable polymers or composites cannot be used for repairs of large bone defects in the cranium [Citation116]. Biodegradable polymer based materials degrade and loose the mechanical strength too fast in relation to the bone regeneration. With regard to degradable metal alloys of magnesium, there are problems in tissue healing due to release of hydrogen gas during degradation process [Citation117].
Thermoset copolymer and the silanized glass fibers form a durable composite for fabrication of patient specific and standard shaped implants [Citation118]. Biocompatibility of FRC implants is the biocompatibility of its components [Citation118–124]. Presence of BG on the implant surface or inside the implant enhance cell maturation of differentiated bone forming cells. In many of the FRC implant studies, there have been BG (S53P4) particles in the FRC implant [Citation120–124]. BGs are synthetic dissolving biocompatible osteoconductive-osteoinductive bone substitutes. Some compositions (S53P4) of BGs have clinically been used because of antibacterial and angiogenesis-promoting properties [Citation125–137]. Antimicrobial efficiency has been shown for more than 20 microbe species, including Staphylococcus aureus and Staphylococcus epidermis, which are the most common pathogens in periprosthetic infections. Clinical studies with cranial FRC-BG implants have been for improving osteogenesis, angiogenesis and antimicrobial properties and long term protection of brain tissues [Citation115,Citation138,Citation139].
In the biological environment ions of calcium and phosphorus are released from the BG and they biomineralize on the material surface, like the surface of glass FRC-BG implant [Citation130]. For cells, at the early stage of osteogenesis, released ions from the BG and slightly increased pH due to ion exchange reactions are inducing differentiation of mesenchymal stem cells to cell lines for bone formation. This, in conjunction with biomineralization promotes bone growth. With regard to osseointegration, i.e. bonding between the BG of the implant and bone tissue, a series of reactions starting at the glass surface followed by a series of biological reactions are occurring. The different reaction steps taking place at the glass surface depend mainly on the glass composition but also on the surface topography, surface area of glass, and flow of the interstistial fluid in the microenvironment close to the glass surfaces. In the subsequent steps, calcium and phosphate from the solution, and migrating from the bulk glass, form first amorphous hydroxyapatite and then crystallize at carbonate substituted hydroxyapatite layer (HA) at the glass surface. This HA layer is compatible with the biological apatite and provides an interfacial bonding between the material and tissue [Citation114]. The present design of FRC-BG cranial implant was approved for clinical use as patient-specific implant and standard shaped implant in Europe in 2014.
Future aspects for the research of FRCs
Use of FRCs in dentistry and medicine has now taken the first steps and the use is increasing rapidly. New applications are tested due to versatile properties of FRC in terms of biomechanics, possibility to add biologically active compounds to the medical device structure and into the polymer matrix. The limitations of biodegradable implants and stem cell based tissue engineering approaches in cranial bone repair can be overcome by using glass FRC-BG implants [Citation140–148]. New applications for FRC will be found from orthopedic and trauma surgery and spine surgery and in more specific dental fields including dental implantology.
Acknowledgements
FRC biomaterial research has been supported by the FRC Research Group of the BioCity Turku Biomaterials and Medical Device Research Program (www.biomaterials.utu.fi). University of Turku, City of Turku, Welfare Division and Turku University Hospital are greatly appreciated.
Disclosure statement
Author is inventor and scientific consultant in the dental FRC material producing Stick Tech Ltd – Member of GC Group. Author has a role also as Member of the Board and shareholder of the Skulle Implants Corporation.
References
- Smith DC. Recent developments and prospects in dental polymers. J Prosthet Dent. 1962;12:1066–1078.
- Vallittu PK. Glass fiber reinforcement in repaired acrylic resin removable dentures: preliminary results of a clinical study. Quintess Int. 1997;28:39–44.
- Narva K. Fibre-reinforced denture base polymers. Clinical performance and mechanical properties. Thesis. Annales Universitatis Turkuensis. University of Turku, 2004.
- Narva KK, Lassila LVJ, Vallittu PK. The static strength and modulus of fiber reinforced denture base polymers. Dent Mater. 2005;21:421–428.
- Narva KK, Lassila LVJ, Vallittu PK. Flexural fatigue of denture base polymer with fiber-reinforced composite reinforcement. Composites, Part A. 2005;36:1275–1281.
- Waltimo T, Luo G, Samaranayake LP, et al. Glass fibre-reinforced composite laced with chlorhexidine digluconate and yeast adhesion. J Mater Sci Mater Med. 2004;15:117–121.
- Ladizesky NH. The Integration of dental resins with highly drawn polyethylene fibres. Clin Mater. 1990;6:181–192.
- Ladizesky NH, Chow TW, Ward IM. The effect of highly drawn polyethylene fibres on the mechanical properties of denture base resins. Clin Mater. 1990;6:209–225.
- Ladizesky NH, Ho CF, Chow TW. Reinforcement of complete denture bases with continuous high performance polyethylene fibers. J Prosthet Dent. 1992;68:934–939.
- Cheng YY, Chow TW. Fabrication of complete denture bases reinforced with polyethylene woven fabric. J Prosthodontics. 1999;8:268–272.
- Ladizesky NH, Chow TW, Cheng YY. Denture base reinforcement using woven polyethylene fiber. Int J Prosthod. 1994;7:307–314.
- Körber HK, Körber S. Experimentelle Untersuchungen zur Passgenauigkeit von GFK-Bruckengerusten ‘Vectris’. Quintess Zahntech. 1998;24:43–53.
- Kolbeck C, Rosentritt M, Behr M, et al. In vitro examination of the fracture strength of 3 different fiber composite and 1 all-ceramic posterior inlay fixed partial denture systems. J Prosthodont. 2002;11:248–253.
- Loose M, Rosentritt M, Leibrock A, et al. In vitro study of fracture strength and marginal adaptation of fiber-reinforced-composite versus all ceramic fixed partial dentures. Eur J Prosthod Rest Dent. 1998;6:55–62.
- Göhring TN, Schmidlin PR, Lutzt F. Two-year clinical and SEM evaluation of glass-fiber-reinforced inlay fixed partial dentures. Am J Dent. 2002;15:35–40.
- Behr M, Rosentritt M, Lang R, et al. Flexural properties of fiber reinforced composite using a vacuum/pressure or a manual adaptation manufacturing process. J Dent. 2000;28:509–514.
- Freilich MA, Karmarker AC, Burstone CJ, et al. Development and clinical applications of a light-polymerized fiber-reinforced composite. J Prosthet Dent. 1998;80:311–318.
- Freilich MA, Duncan JP, Alarcon EK, et al. The design and fabrication of fiber-reinforced implant prostheses. J Prosthet Dent. 2002;88:449–454.
- Freilich MA, Meiers JC, Duncan JP, et al. Clinical evaluation of fiber-reinforced fixed bridges. J Am Dent Assoc. 2002;133:1524–1534.
- Freilich MA, Duncan JP, Meiers JC, et al. Preimpregnated, fiber-reinforced prostheses. Part I. Basic rationale and complete coverage and intracoronal fixed partial denture design. Quintess Int. 1998;29:689–696.
- Ahlstrand WM, Finger WJ. Direct and indirect fiber-reinforced fixed partial dentures: case reports. Quintess Int. 2002;33:359–365.
- Behr M, Hindelang U, Rosentritt M, et al. Comparison of failure rates of adhesive-fixed partial dentures for in vivo and in vitro studies. Clin Oral Invest. 2000;4:25–30.
- Behr M, Rosentritt M, Handel G. Fiber-reinforced composite crowns and FPDs: a clinical report. Int J Prosthod. 2003;15:239–243.
- Göhring TN, Mormann WH, Lutz F. Clinical and scanning electron microscopic evaluation of fiber-reinforced inlay fixed partial dentures: preliminary results after one year. J Prosthet Dent. 1999;82:662–668.
- Göhring TN, Schmidlin PR, Lutz F. Two-year clinical and SEM evaluation of glass-fiber-reinforced inlay fixed partial dentures. American J Dent. 2002;15:35–40.
- Meiers JC, Duncan JP, Freilich MA, et al. Preimpregnated, fiber-reinforced prostheses: Part II. Direct applications: splints and fixed partial dentures. Quintess Int. 1998;29:761–768.
- Malmstrom H, Dellanzo-Savu A, Xiao J, et al. Success, clinical performance and patuient satisfaction of direct fibre-reinforced composite fixed partial dentures: a two-year clinical study. J Oral Rehabil. 2015;42:906–913.
- Meiers JC, Freilich MA. Conservative anterior tooth replacement using fiber-reinforced composite. Oper Dent. 2000;25:239–243.
- Meiers JC, Freilich MA. Chairside prefabricated fiber-reinforced composite fixed partial dentures. Quintess Int. 2001;32:99–104.
- Rosentritt M, Behr M, Lang R, et al. Experimental design of FPD made of all-ceramics and fibre-reinforced composite. Dent Mater. 2000;16:159–165.
- Vallittu PK. The effect of glass fiber reinforcement on the fracture resistance of a provisional fixed partial denture. J Prosthet Dent. 1998;79:125–130.
- Vallittu PK. Prosthodontic treatment with glass fiber reinforced composite resin bonded fixed partial denture. A clinical report. J Prosthet Dent. 1999;82:132–135.
- Dyer SC, Lassila LVJLVJ, Vallittu PK. The effect of internal fiber arrangement on the delamination failure in hybrid composite dental prostheses. J Phys Mesomech. 2004;7:119–122.
- Dyer SR, Lassila LVJ, Jokinen M, et al. Effect of fiber position and orientation on fracture load of fiber-reinforced composite. Dent Mater. 2004;20:947–955.
- Shinya A, Yokoyama D, Lassila LV, et al. Three-dimensional finite element analysis of metal and FRC adhesive fixed dental prosthesis. J Adhes Dent 2008;10:365–371.
- Dyer SR, Lassila LVJ, Alander P, et al. Static strength of molar region direct technique glass-fibre-reinforced composite fixed partial denture. J Oral Rehabil. 2005;32:351–357.
- Özcan M, Breuklander MH, Vallittu PK. Effect of slot preparation on the strength oif glass fiber-reinforced composite inlay retained fixed partial dentures. J Prosthet Dent. 2005;93:337–345.
- Vallittu PK, Shinya A, Baraba A, et al. Fiber-reinforced composites in fixed prosthodontics: Quo vadis? Dent Mater. 2017;33:877–879.
- Wolff D, Wohlrab T, Saure D, et al. Fiber-reinforced composite fixed dental prostheses: a 4-year prospective clinical trial evaluating survival, quality, and effects on surrounding periodontal tissues. J Prosthet Dent. 2018;119:47–52.
- Kumbuloglu O, Özcan M. Clinical survival of indirect, anterior 3-unit surface-retained fibre-reinforced composite fixed dental prosthesis: up to 7.5-years follow-up. J Dent. 2015;43:656–663.
- Butterworth C, Ellakwa AE, Shortall A. A fiber-reinforced composites in restorative dentistry. Dent. Update. 2003;30:300–306.
- Van Dijken JWV, Sunnegård-Grönberg KS. Fiber-reinforced packable resin composites in Class II cavities. J Dent. 2006;34:763–769.
- Garoushi S, Lassila LVJ, Tezvergil A, et al. Load bearing capacity of fibre-reinforced and particlulate filler composite resin combination. J Dent. 2006;34:179–184.
- Garoushi S, Lassila LVJ, Tezvergil A, et al. Fiber-reinforced composite substructure: load bearing capacity of an onlay restoration and flexural properties of the material. J Contemp Dent Pract. 2006;7:1–8.
- Garoushi SK, Lassila LVJ, Vallittu PK. Short fiber reinforced composite: the effect of fiber length and volume fraction. J Contemp Dent Pract. 2006;7:10–17.
- Garoushi S, Vallittu PK, Lassila LVJ. Fracture resistance of short random oriented glass fiber reinforced composite premolar crown. Acta Biomater.2007;3:779–784.
- Garoushi S, Lassila LVJ, Vallittu PK. Direct composite resin restoration of damaged incisors using short fiber-reinforced composite resin. J Dent. 2007;35:731–736.
- Garoushi SK, Ballo AM, Lassila LVJ, et al. Fracture resistance of fragmented incisal edges restored with fiber-reinforced composite. J Adhes Dent. 2006;8:91–95.
- Garoushi S, Lassila LVJ, Tezvergil A, et al. Static and fatigue compression test for particulate filler composite resin with fiber-reinforced composite substructure. Dent Mater. 2007;23:17–23.
- Garoushi SK, Vallittu PK, Watts DC, et al. Polymerization shrinkage of experimental short glass fiber reinforced composite with semi-interpenetrating polymer network matrix. Dent Mater. 2008;24:211–215.
- Garoushi S, Vallittu PK, Lassila LVJ. Fracture toughness, compressive strength and load-bearing capacity of short glass fiber-reinforced composite resin. Chin J Dent Res. 2011;14:1–5.
- Garoushi S, Vallittu PK, Watts DC, et al. Effect of nanofiller fractions and temperature on polymerization shrinkage of glass fiber reinforced filling material. Dent Mater. 2008;24:606–610.
- Garoushi SK, Lassila LV, Vallittu PK. Direct composite resin restoration of an anterior tooth: Effect of fiber-reinforced composite substructure. Eur J Prosthod Rest Dent. 2007;15:61–66.
- Garoushi S, Vallittu PK, Lassila LVJ. Depth of cure and surface microhardness of experimental short fiber-reinforced composite. Acta Odontol Scand. 2008;66:38–42.
- Sewón LA, Ampula L, Vallittu PK. Rehabilitation of a periodontal patient with rapidly progressing marginal alveolar bone loss. A case report. J Clin Periodontol. 2000; 27:615–619.
- Agrawal AA, Chitko SS. The use of silane-coated industrial glass fibers in splinting periodontally mobile teeth. Indian J Dent Res. 2011;22:594–596.
- Özcan M. Kumbuloglu O. Periodontal and trauma splints using fiber reinforced resin composites. Chapter 8. In: Vallittu PK, Özcan M, editors. Clinical guide to principles of fiber-reinforced composites in dentistry. Cambridge (UK): Woodhead Publishing; 2017. p.111–124.
- Mannocci F, Sheriff M, Watson TF, et al. Penetration of bonding resins into fiber posts: a confocal microscopic study. Int Endod J. 2005;38:46–51.
- Mannocci F, Ferrari M, Watson TF. Intermittent loading of teeth restored using quartz fiber, carbon-quartz fiber, and zirconium dioxide ceramic root canal posts. J Adhes Dent. 1999;1:153–158.
- Qualthrough AJ, Chandler NP, Purton DG. A comparison of the retention of tooth coloured posts. Quintess Int. 2003;34:199–201.
- Lassila LV, Tanner J, Le bell AM, et al. Flexural properties of fiber reinforced root canal posts . Dent Mater. 2004;20:29–36.
- LeBell A-M, Tanner J, Lassila LVJ, et al. Bonding of composite resin luting cement to fibre-reinforced composite root canal post. J Adhes Dent. 2004;6:319–325.
- LeBell A-M, Lassila LVJ, Kangasniemi I, et al. Bonding of fibre-reinforced composite post to root canal dentin. J Dent. 2005;33:533–539.
- Le Bell-Rönnlöf AM, Lassila LV, Kangasniemi I, et al. Load-bearing capacity of human incisor restored with various fiber-reinforced composite posts. Dent Mater. 2011;27:107–115.
- Tanner J, Le Bell-Rönnlöf A-M, Vallittu P. Root canal anchoring systems. Chapter 7. In: Vallittu PK, Özcan M editors. Clinical guide to principles of fiber-reinforced composites in dentistry. Cambridge (UK): Woodhead Publishing; 2017. p. 97–109.
- Ferrari M, Sorrentino R, Juloski J, et al. Post-retained single crowns versus fixed dental prostheses: a 7-year prospective clinical study. J Dent Res. 2017;96:1490–1497.
- Sorrentino R, DiMauro MI, Ferrari M, et al. Complications of endodontically treated teeth restored with fiber posts and single crowns or fixed dental prostheses-a systematic review. Clin Oral Investig. 2016;20:1449–1457.
- Vallittu PK. Are we misusing fiber posts. Guest editorial. Dent Mater. 2016;32:125–126.
- Rantala LI, Lastumaki TM, Peltomaki T, et al. Fatigue resistance of removable orthodontic appliance reinforced with glass fibre weave. J Oral Rehabil. 2003;30:501–506.
- Scribante A, Sfondrini MF. Orthodontic retainers. In: Vallittu PK, Özcan M, editors. Clinical guide to principles of fiber-reinforced composites in dentistry. Cambridge (UK): Woodhead Publishing 2017. p.187–202.
- Özcan M, van der Sleen JM, Kurunmäki H, et al. Comparison of repair methods for ceramic-fused-to-metal crowns. J Prosthodontics. 2006;15:283–288.
- Vallittu PK. High aspect ratio fillers: fiber-reinforced composites and their anisotropic properties. Dent Mater. 2014;31:1–7.
- Vallittu PK. Use of woven glass fibres to reinforce a composite veneer. A fracture resistance and acoustic emission study. J Oral Rehabil. 2002;29:423–429.
- Vallittu PK, Ruyter IE, Ekstrand K. Effect of water storage on the flexural properties of E-glass and silica fiber acrylic resin composite. Int J Prosthod. 1998;11:340–350.
- Vallittu PK. Effect of 180 weeks water storage on the flexural properties of E-glass and silica fiber acrylic resin composite. Int J Prosthodont. 2000;13:334–339.
- Tezvergil A, Lassila LVJ, Vallittu PK. The effect of fiber orientation on the thermal expansion coefficients of the fiber reinforced composites. Dent Mater. 2003;19:471–477.
- Tezvergil A, Lassila LVJ, Vallittu PK. The effect of fiber orientation on the polymerization shrinkage strain of fiber reinforced composite. Dent Mater. 2006;22:610–616.
- Vallittu PK. The effect of void space and polymerisation time on transverse strength of acrylic-glass fiber composite. J Oral Rehabil. 1995;22:257–261.
- Vallittu PK. Impregnation of glass fibers with polymethylmethacrylate using powder-coating method. Appl Compos Mater. 1995;2:51–58.
- Vallittu PK. Some aspects of the tensile strength of unidirectional glass fiber: polymethyl methacrylate composite used in dentures. J Oral Rehabil. 1998;25:100–105.
- Tezvergil A, Lassila LVJ, Vallittu PK. Strength of adhesive-bonded fiber-reinforced composites to enamel and dentine substrates. J Adhes Dent. 2003;5:301–311.
- Tezvergil A, Lassila LVJ, Vallittu PK. The shear bond strength of bidirectional and random-oriented fibre-reinforced composite to tooth structure. J Dent. 2005;33:509–516.
- Vallittu PK, Lassila VP, Lappalainen R. Evaluation of damage to removable dentures in two cities in Finland. Acta Odontol Scand. 1993;51:363–369.
- Vallittu PK, Alakuijala P, Lassila VP, et al. In vitro fatigue fracture of an acrylic resin-based partial denture: an exploratory study. J Prosthet Dent. 1994;72:289–295.
- Vallittu PK, Lassila VP, Lappalainen R. The effect of notch shape and self-cured acrylic repairing on the fatigue resistance of an acrylic resin denture base. J Oral Rehabil. 1996;23:108–113.
- Vallittu PK. Fracture surface characteristics of a damaged acrylic resin based denture as analysed by SEM-technique. J Oral Rehabil. 1996;23:524–529.
- Rosen MR. From treating solution to filler surface and beyond. The life histrory of a silane coupling agent. J Coat Technol. 1978;50:70–82.
- Matinlinna JP, Dahl JE, Karlsson S, et al. The effect of the novel silane system to the flexural properties of E-glass fiber reinforced composites. Silanes Other Coupling Agents. 2009;5:107–121.
- Matinlinna JP, Lassila LVJ, Vallittu PK. Experimental novel silane system in adhesion promotion between dental resin and pretreated titanium. Silicon. 2009;1:249–254.
- Narva K, Vallittu PK, Yli-Urpo A. Clinical survey of acrylic resin removable denture repairs with glass-fiber reinforcement. Int J Prosthodont. 2001;14:219–224.
- Vallittu PK, Sevelius C. Resin-bonded, glass fiber reinforced composite fixed partial dentures: a clinical study. J Prosthet Dent. 2000;84:413–418.
- Xie Q, Lassila LV, Vallittu PK. Comparison of load-bearing capacity of direct resin-bonded fiber-reinforced composite FPDs with four framework designs. J Dent. 2007;35:578–582.
- Nohrström TJ, Vallittu PK, Yli-Urpo A. The effect of position and quantity of glass fibers on the fracture resistance of provisional fixed partial denture. Int J Prosthodont. 2000;13:72–78.
- Sperling LH. Over view of IPNs. Interpenetrating polymer networks. In: Klempner D, Sperling LH, Utracti LA, editors. Advances in chemistry series; 239. Washington, DC: American Chemical Society; 1994. p. 4–6.
- Kallio TT, Lastumäki TM, Vallittu PK. Bonding of restorative and veneering composite resin to some polymeric composites. Dent Mater. 2001;17:80–86.
- Lastumäki T, Lassila LVJ, Vallittu PK. The semi-interpenetrating polymer network matrix of fiber-reinforced composite and its effect on the surface adhesive properties. J Mater Sci Mater Med. 2003;14:803–809.
- Vallittu PK. Interpenetrating polymer networks (IPNs) in dental polymers and composites. J Adhes Sci Technol. 2009;23:961–972.
- van Heumen CCM, vanDijken JWV, Tanner J, et al. Five-year survival of 3-unit fiber-reinforced composite fixed partial dentures in the anterior area. Dent Mater. 2009;25:820–827.
- Tanner J, van Heumen CCM, van Dijeken JWV, et al. Five-year survival of 3-unit fiber-reinforced composite fixed partial dentures in posterior area. Dent Mater. 2010;26:954–960.
- Perea L, Matinlinna JP, Tolvanen M, et al. Fiber-reinforced composite fixed dental prostheses with various pontics. J Adhes Dent. 2014;16:161–168.
- Perea L, Matinlinna JP, Tolvanen M, et al. Monomer priming of denture teeth and its effects on the bond strength of composite resin. J Prosthet Dent. 2014;112:257–266.
- Perea L, Matinlinna JP, Tolvanen M, et al. Penetration depth of monomer systems into acrylic resin denture teeth used as pontics. J Prosthet Dent. 2015;113:480–487.
- Perea L, Matinlinna JP, Tolvanen M, et al. Fracture behavior of pontic of fiber-reinforced composite fixed dental prostheses. Dent Mater J. 2015;34:746–753.
- Özcan M, Vallittu PK. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dent Mater. 2003;19:725–731.
- Wolff D, Geiger S, Ding P, et al. Analysisi of the interdiffusion of resin monomers into pre-polymerized fiber-reinforced composites. Dent Mater. 2012;28:541–547.
- Frese C, Decker C, Rebholz J, et al. Original and repair bond strength of fiber-reinforced compsites in vitro. Dent Mater. 2014;30:456–462.
- Kantola R, Lassila L, Vallittu P. Adhesion of maxillofacial silicone elastomer to a fiber-reinforced composite resin framework. Int J Prosthodont. 2011;24:582–588.
- Kurunmäki H, Kantola R, Hatamleh MM, et al. A fiber-reinforced composite prosthesis restoring a lateral midfacial defect: a clinical report. J Prosthet Dent. 2008;100:348–356.
- Hatamleh MM, Watts DC. Effects of bond primers on bending strength and bonding of glass fibers in fiber-embedded maxillofacial silicone prostheses. J Prosthodont. 2011;20:113–119.
- Hatamleh MM, Watts DC. Effects of accelerated artificial daylight aging on bending strength and bonding of glass fibers in fiber-embedded maxillofacial silicone prostheses. J Prosthodont. 2010;19:357–363.
- Kosor BY, Artunç C, Şahan H. Adhesive retention of experimental fiber-reinforced composite, orthodontic acrylic resin, and aliphatic urethane acrylate to silicone elastomer for maxillofacial prostheses. J Prosthet Dent. 2015;114:142–148.
- Kantola R, Sivén M, Kurunmäki H, et al. Laser doppler imaging of skin microcirculation under fiber-reinforced composite framework of facial prosthesis. Acta Odontol Scand. 2013;72:106–112.
- Brydone AS, Meek D, Maclaine AS. Bone grafting, orthopsedic biomaterials, and the clinical need for bone engineering. Proc Inst Mech Eng H. 2010;225:1329–1343.
- Vallittu PK. Bioactive glass: containing cranial implants: an overview. J Mater Sci. 2017;52:8772–8784.
- Aitasalo KMJ, Piitulainen JM, Rekola J, et al. Craniofacial bone reconstruction with bioactive fiber-reinforced composite implant. Head Neck. 2013;36:722–728.
- Byun SH, Lim HK, Kim SM, et al. The bioresorption and guided bone regeneration of absorbable hydroxyapatite-coated magnesium mesh. J Craniofac Surg. 2017;28:518–523.
- Kim BJ, Piao Y, Wufuer M, et al. Biocompatibility and efficiency of biodegradable magnesium-based plates and screws in the facial fracture model of beagles. J Oral Maxillofac Surg. 2018 [cited 2018 Feb 2]. DOI:10.1016/j.joms.2018.01.015.
- Piitulainen JM, Mattila R, Moritz N, et al. Load-bearing capacity and fracture behaviour of glass fiber-reinforced composite cranioplasty implants. JABFM. 2017;10:e356–e361.
- Posti JP, Piitulainen JM, Hupa L, et al. A glass fiber-reinforced composite: bioactive glass cranioplasty implant: a case study of an early development stage implant removed due to a late infection. J Mech Behav Biomed Mater. 2015;55:191–200.
- Ballo AM, Cekic-Nagas I, Ergun G, et al. Osseointegration of fiber-reinforced composite implants: histological and ultrastructural observations. Dent Mater. 2014;30:e384–e395.
- Ballo AM, Akca EA, Ozen T, et al. Bone tissue responses to glass fiber-reinforced composite implants - a histomorphometric study. Clin Oral Implants Res. 2009;20:608–615.
- Tuusa S, Peltola M, Tirri T, et al. Comparison of two glass fiber-reinforced composite structures as implant material in calvarial bone defect. Bioceramics Key Eng Mater. 2007;361-363:471–474.
- Tuusa SM-R, Peltola MJ, Tirri T, et al. Reconstruction of critical size calvarial bone defect in rabbits with glass-fiber-reinforced composite with bioactive glass granule coating. J Biomed Mater Res. 2008;84:510–519.
- Ballo AM, Kokkari AK, Meretoja VV, et al. Osteoblast proliferation and maturation on bioactive fiber-reinforced composite. J Mater Sci Mater Med. 2008;19:3169–3177.
- Hench LL, West JK. Biological applications of bioactive glasses. Life Chemistry Reports. 1996;13:187–241.
- Hench LL, Xynos ID, Polak JM. Bioactive glasses for in situ tissue regeneration. J Biomater Sci Polym Ed. 2004;15:543–562.
- Välimäki VV, Aro HT. Molecular basis for action of bioactive glasses as bone graft substitute. Scand J Surg. 2006;95:95–102.
- Boccaccini AR, Minay EJ, Krause D. Bioglass coatings on superelastic NiTi wires by electrophoretic deposition (EPD). Electrophoretic Depos Fundam Appl II. Key Eng Mater 2006;314:219–224.
- Ojansivu M, Vanhatupa S, Björkvik L, et al. Bioactive glass ions as strong enhancers of osteogenic differentiation in human adipose stem cells. Acta Biomater. 2015;21:190–203.
- Vallittu PK, Närhi TO, Hupa L. Fiber glass-bioactive glass composite for bone replacing and bone anchoring implants. Dent Mater. 2015;31:371–381.
- Monfoulet LE, Becquart P, Marcaht D, et al. The pH in the microenvironment of human mesenchymal stem cells is a critical factor for optimal osteogenesis in tissue-engineered constructs. Tissue Eng Part A. 2014;20:1827–1840.
- Zhang D, Leppäranta O, Munukka E, et al. Antimicrobial effects and dissolution behaviour of six bioactive glasses. J Biomed Mater Res A. 2010;93:475–483.
- Leppäranta O, Vaahtio M, Peltola T, et al. Antimicrobial effect of bioactive glasses on clinically important anaerobic bacteria in vitro. J Mater Sci: Mater Med. 2008;19:547–551.
- Munukka E, Leppäranta O, Korkeamäki M, et al. Bacterial effects of bioactive glass on clinically important aerobic bacteria. J Mater Sci Mater Med. 2008;19:27–32.
- Lindfors N, Geurts J, Drago L, et al. Antibacterial bioactive glass S53P4, for chronic bone infections: a multinational study. Adv Exp Med Biol. 2017;97:81–92.
- Stoor P, Söderling E, Grenman R. Interactions between the bioactive glass S53P4 and the atrophic rhinitis-associated microorganism klebsiella ozaenae. J Biomed Mater Res. 1999;48:869–874.
- Stoor P, Söderling E, Salonen JI. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol Scand. 1998;56:161–165.
- Piitulainen JM, Kauko T, Aitasalo KMJ, et al. Outcomes of cranioplasty with synthetic materials and autologous bone grafts. World Neurosurg. 2015;83:708–714.
- Piitulainen J, Posti JP, Aitasalo K, et al. Pediatric cranial defect reconstruction using bioactive fiber reinforced composite implant: early outcomes. Acta Neurochir. 2015;157:681–617.
- Thesleff T, Lehtimäki K, Niskakangas T, et al. Cranioplasty with adipose-derived stem cells, beta-tricalcium phosphate granules and supporting mesh: six-year clinical follow-up results. Stem Cells Translat Med. 2017;6:1576–1582.
- Varila L, Lehtonen T, Tuominen J, et al. In vitro behaviour of three biocompatible glasses in composite implants. J Mater Sci Mater Med. 2012;23:2425–2435.
- Athanasiou KA, Agrawal CM, Barber FA, et al. Orthopaedic applications for PLA-PGA biodegradable polymers. Arthroscopy. 1998;14:726–737.
- Middleton JC, Tipton AJ. Synthetic biodegradable polymers as orthopaedic devices. Biomaterials. 2000;21:2335–2346.
- Meyer F, Wardale J, Best S, et al. Effects of lactic acid and glycolic acid on human osteoblasts: a way to understand PLGA involvement in PLGA/calcium phosphate composite failure. J Orthop Res. 2012;30:864–871.
- Ignatius AA, Claes LE. In vitro biocompatibility of bioresorbable polymers: poly(L, DL-lactide) and poly(L-lactide-co-glycolide). Biomaterials. 1996;17:831–839.
- Pihlajamäki H, Salminen S, Laitinen O, et al. Tissue response to polyglycolide, polydioxanone, polylevolactide, and metallic pins in cancellous bone: an experimental study on rabbits. J Orthop Res. 2006;24:1597–1606.
- Böstman O, Pihlajamäki H. Adverse tissue reactions to bioabsorbable fixation devices. Clin Orthop Relat Res. 2000;(371:):216–227.
- Barber FA, Dockery WD. Long-term absorption of poly-L-lactic Acid interference screws. Arthroscopy. 2006;22:820–826.