ABSTRACT
The ecological health of River Ringim, Jigawa State, northwestern Nigeria was assessed between November 2018 and March 2019. Key physico-chemical variables alongside macroinvertebrates assemblage were used to achieve the aim of the study. The result of the physico-chemical variables showed that the four stations sampled were relatively perturbed judging from the high values of turbidity, nitrate, phosphate, BOD, TDS, and EC. Twelve (12) taxa and 365 individuals of macroinvertebrates were recorded in the entire study period of five months, confirming the deteriorating state of the river system. Hemiptera which was represented by 126 individuals was the most preponderant macroinvertebrate Order, followed by Coleoptera with 100 individuals. The least represented Order was Crustacea with only five individuals. The result of the diversity indices conducted showed that the Margalef index (1.32), Simpson diversity (0.76), and Shannon-Weiner index (1.59) were highest in Station 1. The results were used to check the implementation of the Chanchaga multimetric index and it showed that that the four stations of River Ringim were of poor water quality with a corresponding D ecological category. This further, confirmed the deteriorating state of the four stations sampled. We recommend that macroinvertebrate-based multimetric index (MMI) be developed for the river using a macroinvertebrates particular to the river instead of using the results in this study in checking the implementation of MMI developed for another river. This will ascertain the actual level of degradation River Ringim is undergoing due to the numerous human disturbances within the river catchments.
Introduction
Human influences have affected tremendously most of the world’s ecosystems ranging from reduction of biotic assemblages across terrestrial and aquatic ecosystems and deterioration of the quality of the ecosystems (Ge et al., Citation2022; Guo, Lenoir, & Bonebrake, Citation2018; Keke et al., Citation2021; Ogidiaka, Ikomi, Akamagwuna, & Edegbene, Citation2022). In recent times, freshwater ecosystems’ biotic assemblages have been on the decline owing to incessant human activities within the catchments and watersheds of these systems (Keke et al., Citation2021). The declining state of the freshwater biota results from multiple pressures including climate change, land use types, and channel alteration (Ge et al., Citation2022; Liu et al., Citation2021). In reducing the negative effects of these dangers befalling freshwater ecosystems globally, different nations of the world have come up with water policy frameworks to protect water bodies and their resources. For instance, Europe has come up with the European Water Framework Directive (WFD) to guide the activities of humans within the water systems (European Commission, Citation2000). Other countries like the United States of America and South Africa have also implemented water directives and frameworks serving as guides for water managers to forestall the degradation of water systems within their jurisdictions (González-Paz, Delgado, & Pardo, Citation2022). The major aim of the implementation of water directives and frameworks is for water systems to attain healthy ecological status.
The healthy ecological status of the water system is adjudged by the water quality and taxonomic and functional assemblages of biotic components of the system (Solak et al., Citation2020). Ecological status is achieved by evaluating environmental variables and biological constituents (e.g. phytoplankton, zooplankton, macroinvertebrates, and fish species). Environmental variables (e.g. physico-chemical) are commonly employed in determining the quality of sampling stations of water systems under investigation (Edegbene et al., Citation2022; Edegbene, Elakhame, Arimoro, Osimen, & Odume, Citation2019; Garba, Ogidiaka, Akamagwuna, Nwaka, & Edegebene, Citation2022). These variables have been employed for the assessment of ecological status/health of freshwater systems at both spatial and temporal scale in different studies (Arimoro, Obi-Iyeke, & Obukeni, Citation2012; Arimoro, Odume, Uhunoma, & Edegbene, Citation2015; Omovoh et al., Citation2022). However, the combination of the use of physico-chemical variables alongside biological assemblages gives a vivid picture of the ecological status/health of water systems. In choosing biological components for assessing the ecological status/health of water systems, several factors are weighed, namely; level of sensitivity to local disturbances, easy sampling, diversity, life cycle pattern, morphological structure, and easy identification of taxa (Ogidiaka, Ikomi, Akamagwuna, & Edegbene, Citation2022). Among the biota used in assessing ecological status/health, macroinvertebrates are one of the most explored as they possess most of the aforementioned factors (Amelework, Getachew, Minwyelet, & Amare, Citation2021; Odabaşi et al., Citation2022).
Several macroinvertebrate biological metrics have been developed to assess the ecological status/health of freshwater systems. These metrics include taxonomy, traits, and diversity indices (Ge et al., Citation2022; Odabaşi et al., Citation2022). Multiple macroinvertebrate metrics have been combined and integrated into a single score called a multimetric index (Amelework, Getachew, Minwyelet, & Amare, Citation2021; Odabaşi et al., Citation2022). For instance, Odabaşi et al. (Citation2022) integrated four out of the 10 candidate metrics into a multimetric index for Sakarya River, Turkey, and they adjudged the multimetric index to be effective for the biological monitoring of similar river systems within Turkey. Also, Amelework et al. (Citation2021) developed a multimetric index using macroinvertebrates to assess the ecological health of selected rivers in Ethiopia, and they integrated five out of 30 candidate metrics selected for the multimetric index developed for the rivers. Amelework et al. (Citation2021) recommended that the multimetric index they developed was effective in assessing and monitoring different Afrotropical Rivers.
Following the thinking of Amelework et al. (Citation2021) and Odabaşi et al. (Citation2022), we assessed the ecological health of River Ringim in the Northwestern region of Nigeria using the Chanchaga multimetric index (MMIchanchaga) developed by Edegbene et al. (Citation2019) in a bid to ascertain the level of deterioration the river system is subjected to. As we have earlier mentioned, owing to the fact that other authors have argued that the combination of physico-chemical variables and biological community is more effective in arriving at a conclusion on the current status of the water systems, we adopted a combination of physico-chemical variables, macroinvertebrates assemblage, and diversity together with the MMIchanchaga to assess the ecological health of River Ringim, Northwestern region of Nigeria. Therefore, the aim of the present study was to assess the ecological health of River Ringim using physico-chemical variables and macroinvertebrates assemblage. We also checked the implementation of the macroinvertebrate-based Chanchaga multimetric Index (MMIchanchaga) on the river using macroinvertebrate abundance data.
Materials and methods
Study area
River Ringim is located in the Ringim Local Government Area of Jigawa State, Nigeria. The river is within the interception of latitude 12°17”N 9°28”E and longitude 12.283°N 9.467°E of the equator (). The river is a tributary of River Wudil located in the Kano State of Nigeria. River Ringim flows through Ringim town to the Hadejia River where it splits into three channels in the Hadejia Nguru Wetland: The Marma channel which flows into the Nguru Lake, the old Hadejia River which joins up with the Jama’are River to become the Yobe River and the relatively small Burum Gana River. The vegetation of the study area is dominated by sparsely distributed trees and majorly shrubs particularly in a Sudan Savanah belt (Edegbene et al., Citation2022). The study area’s climate is typical of a tropical zone with a mean yearly temperature of 37°C characterized by two seasons (dry and wet; Garba et al., Citation2022; Ghali et al., Citation2020). The wet season is relatively short which takes place between June and September, and the dry season is between October and May.
For the purpose of this study well marked out four stations were selected based on the level of anthropogenic activities within the river catchments (). Station 1 was located in the Yakasawa community, and the stream bed is mainly loam and clay. The bank of this station is dominated by typha grass. Activities within the station include subsistence farming and fishing. Station 2 is about 2 km away from Station 1. The streambed is dominated with loam and silt, and mango and guava trees dominate the vegetation within the station by few shrubs. The anthropogenic activities here include farming, transportation, washing, dredging of land, and bathing. Station 3 is located in Zangon Kanya village which is about 1 km away from Station 2. The streambed is mainly sandy and loamy. Shrubs and typha grass dominate the vegetation in this station. Sand dredging, defecation, farming, and bathing are among the anthropogenic activities in the station. Station 4 is located within the Yandutse community and is about 1.5 km away from Station 3. The streambed is made up of mainly loam and mud. Neem trees (Azadirachta indica) dominate this station with sparse distribution of shrubs. Open defecation, farming, and fishing are common human activities within the station.
Sample collection
Physico-chemical variables sampling and analyses
Sampling was carried out monthly for a period of 5 months between November 2018 and March 2019. The following are the physico-chemical variables analyzed in the course of the present study: air and water temperature, water depth, flow velocity, transparency, pH, electrical conductivity (EC), turbidity, total dissolved solids (TDS), nitrate, phosphate, dissolved oxygen (DO) and biological demand oxygen (BOD). Air and water temperature were measured using a mercury-in-glass thermometer in oC. Water depth was measured using a calibrated stake in centimeters and later converted to meters after measurement. Flow velocity was measured by timing a floated ball in three trials (Gordon, McMahon, & Finlayson, Citation1994). The average of the three trials was taken as the flow velocity of each of the stations sampled, and it was measured in meter per second (m/s). Transparency and pH were measured using seechi disc and pH meter (model: HANNA HI 9828), respectively. Electrical conductivity and TDS readings were taken with the aid of a conductivity meter (model: DDSJ-308A). Turbidity was measured using a turbidity meter (model: WGZ-B) and it was measured in a nephelometric turbidity unit (NTU). Nitrates, phosphate, DO and BOD were determined following APHA (American Public Health Association (Citation1998)) methods.
Macroinvertebrates sampling, sorting, and identification
Concurrently, as physico-chemical variables were sampled on every sampling occasion, macroinvertebrates were also sampled. The Kick sampling method was employed in sampling macroinvertebrates. A modified kick net of 500 µm mesh size was towed against the water current within an approximately 40 m wadeable portion of all the different streambeds present in each station. Sampling at each streambed was done for 3 minutes. Macroinvertebrates collected in all the streambed per station were pooled together as one composite sample. After which, preliminary sorting was done at the field by pouring all collected samples per station on a white enamel tray and forceps were used to pick out moveable organisms into a container containing 10% formalin. The unsorted samples together with the already sorted samples were transported to the laboratory for final sorting and identification using a white enamel tray, forceps, hand lens, microscope, and identification guides present in Nigeria (Arimoro & James, Citation2008; Arimoro, Obi-Iyeke, & Obukeni, Citation2012). This was supplemented with an identification guide elsewhere (de Moore, Day, & de Moore, Citation2003). A macroinvertebrate taxonomic expert among the coauthors also confirmed most of the macroinvertebrates that were collected during the study.
Data analyses
Descriptive statistics (i.e. mean and standard deviation) for all the physico-chemical variables per station were computed using Paleontological Statistical Package (PAST) (Hammer, Harper, & Ryan, Citation2001). Analysis of variance (ANOVA) was performed to elucidate the significant differences in physico-chemical variables among the four stations sampled. The abundance of the major macroinvertebrates in the entire study period was represented on bar chart. Margalef index, Simpson diversity, evenness index, and Shannon-Weiner diversity were calculated using the diversity function on PAST (Hammer, Harper, & Ryan, Citation2001).
Checking the implementation of the macroinvertebrate-based Chanchaga Multimetric Index (MMIchanchaga)
The Chanchaga multimetric index (MMIchanchaga) was developed using macroinvertebrate taxonomic metrics by Edegbene et al. (Citation2019). The index was developed following four procedures; tests for sensitivity, seasonality and redundancy, and metric integration (Edegbene et al., Citation2019). First, the test for sensitivity or discriminatory potentials of the selected metrics were computed using box and whisker plots to visualize the degree of overlap of the interquartile ranges or medians of each of the selected metrics. This test was performed using Statistica version 13.4.14. Second, the test for seasonality was performed on the metrics that scaled through the test for sensitivity, and the seasonality was computed by multiple comparison of the remaining metrics using Kruskal–Wallis test, and the test was performed using (PAST) (Hammer, Harper, & Ryan, Citation2001). Third, metrics that were seasonally stable were further subjected to test for redundancy, and this was computed using Spearman’s rank correlation coefficient and it was performed using (PAST) (Hammer, Harper, & Ryan, Citation2001). Metrics with r value greater or equal to 0.78 metrics were defined as non-redundant metrics (Edegbene, Elakhame, Arimoro, Osimen, & Odume, Citation2019). Fourth, the metrics that scaled through all the three tests were finally integrated into the multimetric index using a percentile distribution of minimum, lower quartile (25%), mid-quartile (50%), upper quartile (75%) and maximum values of station 1 being that it was the less disturbed station. Details on how the MMIchanchaga was developed can been seen in Edegbene et al. (Citation2019). In all 13 metrics namely Ephemeroptera Plecoptera Trichoptera (%), Ephemeroptera Plecoptera Trichoptera richness, Diptera abundance, Margalef index, Shannon diversity, Coleoptera+Hemiptera abundance, Decapoda abundance, Mollusca abundance, Odonata abundance, Coleoptera+Hemiptera (%), Decapoda (%), Hemiptera+ Diptera richness and Chironomidae+Oligochaeta (%) were retained and integrated into MMIchanchaga ( according to Edegbene et al., Citation2019) for the selected metrics statistics and scores. The metric statistics and scores were used in checking the implementation of the macroinvertebrate-based MMIchanchaga as well as the ecological health of River Ringim. For the definition of the 13 metrics used in the present study, see according to Edegbene et al. (Citation2019).
Table 1. Summary of physico-chemical variables in the stations sampled.
Table 2. Diversity indices of macroinvertebrates in the stations sampled.
Table 3. Macroinvertebrate metrics for River Ringim as per the definition for the 13 selected metrics according to of Edegbene (2019).
Table 4. Ecological health of the sampled stations of River Ringim.
Results
Physico-chemical variables
shows the mean, standard deviation and level of significant differences of physico-chemical variables among the sampling stations in River Ringim. The highest mean air temperature (24.4 ± 1.67°C), water temperature (22.4 ± 1.25°C) and flow velocity (0.56 ± 0.052 m/s) were recorded in Station 4. Station 3 was more transparent (5.3 ± 0.98 cm) than the remaining three stations. Further, turbidity (182.58 ± 5.07 NTU), nitrate (1.35 ± 0.52 mg/l) and phosphate (1.31 ± 0.24 mg/l) were highest in Station 3. Water depth (1.18 ± 0.14 m), pH (6.8 ± 0.39), EC (125.48 ± 6.22 µS/cm), TDS (77.18 ± 4.98 mg/l) and DO (0.57 ± 0.24 mg/l) were highest in Station 2. BOD (0.22 ± 0.115 mg/l) was highest in Station 1. Water depth, flow velocity, turbidity, nitrate, and phosphate showed significant differences among the stations sampled (P < 0.05). The remaining physico-chemical variables showed no significant differences among the stations sampled (P > 0.05).
Abundance of major macroinvertebrates in the study area
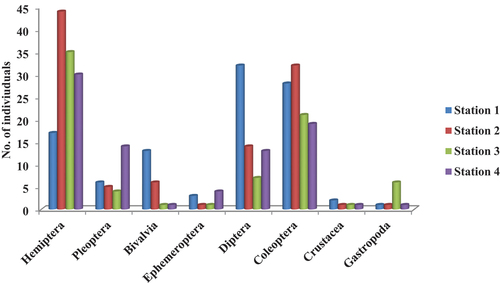
A total of 12 taxa and 365 macroinvertebrates individuals were recorded in the entire study period. Hemiptera with a total of 126 individuals was the most preponderant Order, and it was mostly represented in Station 2 with 44 individuals (). This was immediately followed by Coleoptera represented by a total of 100 individuals, and the highest Coleoptera was in Station 2 with 32 individuals (). The least abundant macroinvertebrate was Crustacea represented by five individuals in all the four stations sampled ().
Diversity of macroinvertebrates in the stations sampled
summarizes the diversity indices of macroinvertebrates in the sampling stations of River Ringim. Margalef index was highest in Station 1 (1.32) and lowest in Station 4 (0.70). Simpson diversity was highest in Station 1 (0.76) and lowest in Station 3 (0.66). Station 4 (0.93) was more even in macroinvertebrate distribution, and the lowest was in Station 1 (0.70). Shannon-Weiner index was highest in Station 1 (1.59) and lowest in Station 3 (1.28).
Ecological health of River Ringim
The metrics of the macroinvertebrates collected in River Ringim were calculated based on the definition of each of the metrics in of Edegbene et al. (Citation2019). All the 13 metrics integrated into the Chanchaga multimetric index (MMIchanchaga) were represented in the present study area except, Decapoda abundance and Decapoda (%), hence they were assigned 0 scores in the four stations sampled ().
Following the statistics and their corresponding scores of in Edegbene et al. (Citation2019), the following scores were awarded to the 13 metrics of macroinvertebrates in the present study. EPT (%), EPT richness, Margalef index, Decapoda abundance, Odonata abundance, and Decapoda (%) were awarded a score of 1 each for Stations 1–4 (). Shannon diversity and Chironomidae+ Oligochaeta (%) were awarded a score of 3 each for Stations 1–4 (). Coleoptera + Hemiptera abundance and Coleoptera + Hemiptera (%) were awarded a score of 5 each for Stations 1–4 (). Diptera abundance was awarded a score of 1 in Stations 1, and 3 each in Stations 2–4 (). Hemiptera + Diptera richness was awarded a score 3 in Stations 2, and 5 each in Stations 1, 3 and 4 ().
Judging from Table 5 in Edegbene et al. (Citation2019), the corresponding MMIchanchaga scores for Stations 1, 2, 3, and 4 were 29, 29, 31, and 35, respectively (). This showed that all the four stations sampled in River Ringim were of poor water quality condition/river health condition, corresponding to D ecological category ().
Discussion
The ecological health of River Ringim, Northwestern, Nigeria was assessed using physico-chemical variables, macroinvertebrates assemblage, and diversity in a bid to unravel the level of degradation the system has been subjected to. We also checked the implementation of the macroinvertebrate-based Chanchaga Multimetric Index (MMIchanchaga) on the ecological health of the river. To achieve this, we first explored the level of degradation of the stations sampled in the river system from the result of the analyses of physico-chemical variables. Proportionately high values of physico-chemical variables were recorded in the four stations, most especially turbidity, nitrate, phosphate, and EC. The high values of most of the physico-chemical variables recorded in the present study were expected owing to the level of human activities within the catchments of the studied stations. Our findings corroborate with recent studies within the Northwestern region of Nigeria (e.g. Edegbene et al., Citation2022; Garba et al., Citation2022). Edegbene et al. (Citation2022) reported concomitant high values of phosphate and EC typical of a deteriorating system in River Kafin Hausa, northwestern Nigeria. They asserted that the high importance of these variables resulted from the continuous human influences within the river catchments such as indiscriminate defecation, agricultural activities, and over-dependent on the river for household use.
On the other hand, stressors such as urbanization, industrialization, and agricultural development have also been implicated to be the cause of river systems degradation in Sub-Saharan Africa, Nigeria inclusive (Keke et al., Citation2021; Ogidiaka et al., Citation2022). These activities were vividly present in the catchments of River Ringim, further confirming the reason for the high values of some pollution indicating physico-chemical variables. Keke et al. (Citation2021) have alluded to the fact that the localization of market and industrial undertakings aggravated the level of environmental variables in most of the sites they sampled in a river in the southern part of Nigeria. The concentrations of environmental variables indicate factors that pattern the assemblages of macroinvertebrates in aquatic systems. Hence, we further explored the use of macroinvertebrates assemblage and diversity in the riverine system.
In the present study, we recorded only 365 macroinvertebrates individuals, which is quite low compared to similar studies in Nigeria and elsewhere (e.g. Dzavi, Menbohan, Mboye, Nwaha, & A Ngon, Citation2022; Garba et al., Citation2022; Omovoh et al., Citation2022). Dzavi et al. (Citation2022) recorded 13,690 macroinvertebrates individuals in selected forested streams in Cameroun. They attributed the wide assemblage of macroinvertebrates to relatively stable environmental conditions. Dallas (Citation2007) also attributed the multiplication of macroinvertebrates to stable environmental conditions such as forested catchments, good environmental conditions, and fewer human influences. These favorable conditions were lacking in the present study area, as the catchments are typical of the Sudan savannah vegetation belt of Nigeria characterized by the sparse distribution of trees and shrubs unlike in the case of the study areas by Dzavi, Menbohan, Mboye, Nwaha, and A Ngon (Citation2022) and Dallas (Citation2007). The abundance and diversity of macroinvertebrates are predicated on land use patterns within riverine systems. Suárez, Barrios, and Teixeira de Mello (Citation2022) recently reported that aquatic biota responded on a spatial scale to environmental stressors like urban, agricultural, and other land use types. Other authors have stressed that in most cases urban and agricultural activities impact negatively the assemblage and diversity of macroinvertebrates and the impact is long term and in most cases they are irreversible (Sabater, Elosegi, & Ludwig, Citation2018; Walsh, Jack, Cottingham, Groffman, & Morgan, Citation2005). This was noticed in the present study as we recorded very low abundance and diversity of macroinvertebrates in all the stations sampled in the present study area. This presages that the continuous human influence observed in the studied stations may be happening for a long time and probably irreversible owing to the continuous record of low macroinvertebrates individuals recorded monthly in the four stations during the five months’ study period. This calls for proper management measures to be taken by the concerned authorities to avoid the local extinction of the major macroinvertebrates population in River Ringim.
Judging from the relatively high values of physico-chemical variables and assemblage of macroinvertebrates in the four stations sampled, it is clear that River Ringim is heavily polluted. Further confirming the ecological health of the river is our result in checking the implementation of MMIchanchaga developed by Edegbene et al. (Citation2019) which showed that the river is indeed heavily polluted as the river health condition of the four stations sampled was poor with a corresponding D ecological category. It has been confirmed that the classification of riverine systems into ecological health status will benefit from multilayered approaches including both environmental and biotic indices (González-Paz, Delgado, & Pardo, Citation2022). González-Paz, Delgado, and Pardo (Citation2022) reported that nutrient enrichment, biotic model, and multimetric index showed a varied level of interpretation of the ecological status across river typologies in Spain. This corroborates our result in the present study, as all ecological indicators used in assessing the health of the four stations sampled, showed that they were heavily polluted. This account for the specificity of the responses of macroinvertebrates to various stressors within the catchments of River Ringim.
Conclusions and recommendations
Our present study gives a vivid level of pollution in the four stations of River Ringim. Results from the analyses of key physico-chemical variables showed an increasingly degrading system. Further, the very low number of macroinvertebrates recorded in the study is also a pointer to the deteriorating states of the four stations sampled. Further confirming our assertion are the results we obtained from checking the implementation of MMIchanchaga developed by Edegbene et al. (Citation2019). The four stations were of poor water quality condition with a corresponding ecological category of D. To further confirm the result of the present study, we recommend that a more detailed study be carried out along the stretch of River Ringim and its tributaries within Jigawa and Kano States of Nigeria. Also, a macroinvertebrate-based multimetric index (MMI) should be developed for the river using macroinvertebrates particular to the river instead of checking the implementation of another MMI developed for another river, this will ascertain the actual level of degradation River Ringim is undergoing due to the numerous human disturbances within the river catchments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
References
- Amelework, Z., Getachew, B., Minwyelet, M., & Amare, M. (2021). Development of a multimetric index for assessing the ecological integrity of some selected rivers and streams in the north-eastern part of Lake Tana sub-basin, Ethiopia. African Journal of Aquatic Science. doi:10.2989/16085914.2021.1933375
- APHA (American Public Health Association). (1998). Standard methods for the examination of water and Wastewater, WEF and AWWA (20th ed.). Washington DC, USA: American Public Health Association.
- Arimoro, F. O., & James, H. M. (2008). Preliminary pictorial guide to the macroinvertebrates of Delta State Rivers, Southern Nigeria. Grahamstown: Albany Museum.
- Arimoro, F. O., Obi-Iyeke, G. E., & Obukeni, P. J. O. (2012). Spatiotemporal variation of macroinvertebrates in relation to canopy cover and other environmental factors in Eriora River, Niger Delta, Nigeria. Environmental Monitoring and Assessment, 184, 6449–6461. doi:10.1007/s10661-011-2432-9
- Arimoro, F. O., Odume, N. O., Uhunoma, S. I., & Edegbene, A. O. (2015). Anthropogenic impact on water chemistry and benthic macroinvertebrate associated changes in a Southern Nigeria stream. Environmental Monitoring and Assessment, 187(2), 14. doi:10.1007/s10661-014-4251-2
- Dallas, H. F. (2007). The effect of biotope-specific sampling for aquatic macroinvertebrates on reference site classification and the identification of environmental predictors in Mpumalanga, South Africa. African Journal of Aquatic Science, 32, 165–173. doi:10.2989/AJAS.2007.32.2.8.205
- de Moore, I. J., Day, J. A., & de Moore, F. C. (2003). Guides to freshwater invertebrates of Southern Africa, Volume 9. Water Research Commission Report No. TT 207/03. 288pp
- Dzavi, J., Menbohan, S. F., Mboye, B. R., Nwaha, M., & A Ngon, E. B. (2022). Spatiotemporal Variation of benthic macroinvertebrates in some tropical forest streams of the Nyong Catchment (Cameroon). Open Journal of Applied Science, 12(07), 1210–1231. doi:https://doi.org/10.4236/ojapps.2022.127082
- Edegbene, A. O., Abdullahi, Y., Akamagwuna, F. C., Omovoh, B. O., Osimen, E. C., & Ogidiaka, E. (2022). Are zooplankton useful indicators of ecological quality in Afrotropical Ephemeral River impacted by human activities? Environmental Monitoring and Assessment, 194(6), 399. doi:https://doi.org/10.1007/s10661-022-10061-4
- Edegbene, A. O., Elakhame, L. A., Arimoro, F. O., Osimen, E. C., & Odume, O. N. (2019). Development of macroinvertebrate multimetric index for ecological evaluation of a river in North Central Nigeria. Environmental Monitoring and Assessment, 191(5), 274. doi:10.1007/s10661-019-7438-8
- European Commission. (2000). Directive 2000/60/EC of the European Parliament and of the Council of 23 of October 2000 establishing a framework for community action in the field of water policy. Official Journal of European Communication, 327, 1–73.
- Garba, F., Ogidiaka, E., Akamagwuna, F. C., Nwaka, K. H., & Edegebene, A. O. (2022). Deteriorating water quality state on the structural assemblage of aquatic insects in a North-Western Nigerian River. Water Science, 36(1), 22–31. doi:10.1080/23570008.2022.2034396
- Ge, Y., Liu, Z., García-Gir´on, J., Chen, X., Yan, Y., Li, Z., & Xie, Z. (2022). Human-induced loss of functional and phylogenetic diversity is mediated by concomitant deterministic processes in subtropical aquatic insect communities. Ecological Indicators, 136, 108600. doi:10.1016/j.ecolind.2022.108600
- Ghali, H., Osimen, E. C., Ogidiaka, E., Akamagwuna, F. C., Keke, U. N., & Edegbene, A. O. (2020). Preliminary assessment of the deteriorating state of an irrigation dam in north western Nigeria using phytoplankton structural assemblage and environmental factors. Water Science, 34(1), 181–189. doi:10.1080/11104929.2020.1816152
- González-Paz, L., Delgado, C., & Pardo, I. (2022). How good is good ecological status? A test across river typologies, diatom indices and biological elements. The Science of the Total Environment, 815, 152901. doi:http://dx.doi.org/10.1016/j.scitotenv.2021.152901
- Gordon, N. D., McMahon, T. A., & Finlayson, B. L. (1994). Stream Hydrology, an introduction for Ecologists. New York: John Wiley & Sons Ltd, 526Pp.
- Guo, F., Lenoir, J., & Bonebrake, T. C. (2018). Land-use change interacts with climate to determine elevational species redistribution. Nature Communication, 9(1). doi:10.1038/s41467-018-03786-9
- Hammer, Ø., Harper, D. A. T., & Ryan, P. D. (2001). PAST: Paleontological statistics software package for education and data analysis. Palaeontologia Electronica, 4(1), 9. http://palaeo-electronica.org/2001_1/past/issue1_01.htm. Accessed 1 July 2015
- Keke, U. N., Omoigberale, M. O., Ezenwa, I., Yusuf, A., Biose, E., Nweke, N., Edegbene AO, & Arimoro, F. O. (2021). Macroinvertebrate communities and physicochemical characteristics along an anthropogenic stress gradient in a southern Nigeria stream: Implications for ecological restoration. Environmental Sustainability, 12, 100157. doi:10.1016/j.indic.2021.100157
- Liu, Z., Zhou, T., Cui, Y., Li, Z., Wang, W., Chen, Y., & Xie, Z. (2021). Environmental filtering and spatial processes equally contributed to macroinvertebrate metacommunity dynamics in the highly urbanized river networks in Shenzhen, South China. Ecological Process, 10(1), 23. doi:10.1186/s13717-021-00297-2
- Odabaşi, D. A., Odabaşı, S., Ergül, H. A., Özkan, N., Boyaci, Y. O., Bayköse, A., Kayal, M, Ekmekçi, F, Dağdeviren, M, Güzel, B, & Dügel, M. (2022). Development of a macroinvertebrate‑based multimetric index for biological assessment of streams in the Sakarya River Basin, Turkey. Biologia, 77(5), 1317–1326. doi:https://doi.org/10.1007/s11756-022-01041-7
- Ogidiaka, E., Ikomi, R. B., Akamagwuna, F. C., & Edegbene, A. O. (2022). Exploratory accounts of the increasing pollution gradients and macroinvertebrates structural assemblage in an Afrotropical Estuary. Biologia, 77(8), 2103–2114. doi:10.1007/s11756-022-01076-w
- Omovoh, B. O., Arimoro, F. O., Anyanwale, A. V., Egwim, E. C., Omovoh, G. O., Akamugwuna, F. C., Zakari H, & Edegbene, A. O. (2022). Macroinvertebrates of Wupa River, Abuja, Nigeria: Do environmental variables pattern their assemblages? Biology Insight, 1, 1–9. doi:10.55085/bi.2022.612
- Sabater, S., Elosegi, A., & Ludwig, R. (2018). Multiple stressors in river ecosystems. In S. Sabater, A. Elosegi, & R. Ludwig (Eds.), Status, impacts and prospects for the future (pp. 404). Amsterdam: Elsevier.
- Solak, C. N., Peszek, L., Yilmaz, E., Ergül, H. A., Kayal, M., Ekmekçi, F., Várbíró, G, Yüce, AM, Canli, O, Binici, MS, & Ács, E. (2020). Use of diatoms in monitoring the Sakarya River basinTurkey. Water, 12(3), 703. doi:https://doi.org/10.3390/w12030703
- Suárez, B., Barrios, M., & Teixeira de Mello, F. (2022). Macroinvertebrates’ response to different land use in lowland streams from Uruguay: Use of artificial substrates for biomonitoring. Neotropical Biodiversity, 8(1), 136–146. doi:10.1080/23766808.2022.2049178
- Walsh, C. J., Jack, W. F., Cottingham, P. D., Groffman, P. M., & Morgan, R. P. (2005). The urban syndrome: Current knowledge and the search for a cure. Journal of North America Benthological Society, 24(3), 706–723. doi:10.1899/04-028.1