Abstract
With the success of upper extremity and face transplantation, the field of vascularized composite allotransplantation (VCA) is preparing to expand into other reconstructive areas of the body. Lower extremity allotransplantation has the potential to offer patients improved function over current prosthetics, reduce phantom limb pain, and reduce prosthetic associated complications. However, these benefits must be weighed against the obstacles of slow-paced nerve regeneration, difficult perioperative management, and lifelong immunosuppression. Four cases of lower extremity transplantation have been performed with increasingly high-risk operations, marked by deaths of the last 2 recipients. As lower extremity allotransplantation proceeds, selecting the appropriate candidates to optimize outcomes is critical. We propose a classification system of lower extremity allotransplantation based on the extent of preservation of recipient muscular innervation and detail a practical next recipient. If a safe and thoughtful approach is taken, we believe lower extremity allotransplantation can achieve similarly positive results to those seen in hand and face allotransplantation.
Introduction
Vascularized composite allotransplantation (VCA) has established itself as a new rung in the reconstructive ladder. Upper extremity and face transplants are occurring on a more regular basis. Functional outcomes have been more impressive, faster paced, and have continued to improve over longer periods of time than previously expected.Citation1-4 With the relative success of early vascularized composite allotransplants, newer avenues are being explored. Boundaries are being tested in terms of combined hand/face and hand/leg transplants, and more novel anatomic regions are being considered for transplantation.Citation5-11
The natural progression of the field is to explore the role of lower extremity allotransplantation.Citation12 In 2005, an estimated 1,027,000 people lived with loss of at least one lower limb, 20% of which were not from dysvascular disease.Citation13 These numbers are projected to more than double by 2050. As of December 2013, 1,558 wounded warriors have returned from Iraq or Afghanistan with a major limb amputation.Citation14 A recent survey by Carty et al. indicated that a significant portion (43%) of lower limb amputees are interested in being evaluated for lower extremity allotransplantation.Citation15 High above knee amputations and/or concomitant upper extremity amputations make prosthetic use difficult and, at times, impractical. Poor prosthetic fit can result in recurrent pressure sores and pain, again preventing their use.Citation16-19 Knowledge gained from upper extremity transplantation will inform early cases of lower extremity transplantation.
The level of functional recovery required for restoration of gait and balance is currently unknown, and will only be elucidated from human patients, as there are no animals to simulate the bipedal gait of humans.Citation20 As with upper extremity transplantation, the risks of lifelong immunosuppression must be weighed against the function offered from current and future prosthetics. We aim to review the challenges and benefits of lower extremity allotransplantation, and through the examination of cases performed to date, attempt to construct a framework for recipient selection. We emphasize the need to take small predictable steps while the field advances, as was done with upper extremity and facial transplantation.
Lower Extremity Transplantation Cases
Four cases of lower extremity transplantation have been carried out to date ().Citation5-8,Citation21-24 Each case is outlined with details gathered from all currently available sources, including updates at conferences and media reports.
Table 1. Details of lower extremity transplantation reported to date with outcomes
Case 1
Zuker and colleagues completed the world's first lower extremity transplantation in 2006.Citation5,6 The entire right lower extremity was transplanted between 3 months old ischiopagus twins. The twins were scheduled for separation, but due to inoperable cardiac failure of twin A (donor), emergent separation was initiated to save the life of twin B (recipient). There were 3 lower extremities shared between the twins, forming a ring-like pelvis with radially oriented legs (). In transplanting the right lower extremity from twin A, twin B was provided with 2 normal legs.
Figure 1. Overview of lower extremity transplantation recipients. (A) Illustration of Case 1, ischiopagus twins separated by Zuker et al. with complete lower extremity transplantation from twin A to twin B, including pelvic bone and hip socket. Source: Ref. 6. © Georg Thieme Verlag KG. Reproduced by permission of Georg Thieme Verlag KG. Permission to reuse must be obtained from the rightsholder. (B) Pre- and postoperative photographs and 3D CT reconstructions of Case 2 by Cavadas et al., showing right mid- and left distal femoral allotransplantation. Source: Ref. 7. © 2013 John Wiley and Sons, Inc. Reproduced by permission of John Wiley and Sons, Inc. Permission to reuse must be obtained from the rightsholder. (C) Illustration of recipient from Case 3. Of note, exact levels of amputation/transplantation are unknown due to lack of literature. (D) Preoperative photograph of the recipient from Case 4, demonstrating right shoulder level, left trans-humeral, and bilateral mid-femoral amputation levels. Source: Ref. 22. © 2014 Wolters Kluwer Health. Reproduced by permission of Wolters Kluwer Health. Permission to reuse must be obtained from the rightsholder (reprinted with permission from Nasir S, Kilic YA, Karaaltin MV, et al. Lessons learned from the first quadruple extremity transplantation in the world. Ann Plast Surg 2014; 73(3):336-40; promotional and commercial use of the material in print, digital or mobile device format is prohibited without the permission from the publisher Lippincott Williams & Wilkins. Please contact [email protected] for further information).
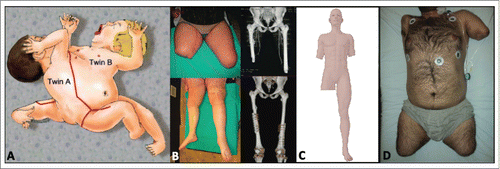
The abnormal anatomy created a complex and intricate procedure, with more to consider than lower extremity allotransplantation between 2 adults with normal anatomy. However, the situation provided a high benefit-to-risk ratio, with no need for postoperative immunosuppression and a high potential for functional rehabilitation given the young age of the recipient. Overall, this created an ideal scenario to proceed with the first lower extremity transplantation, analogous to Dr. Joseph Murray's first kidney transplantation between identical twin brothers.Citation25
Pelvic osteotomies were created to rotate the pelvis along with the hip joint from twin A into the appropriate position to form one pelvis with 2 hip sockets for twin B. The femoral vessels and femoral nerve of twin B perfused and innervated the anterior thigh muscles of the third limb, which were preserved to provide increased knee extension function to the donor limb. The donor leg vessels were dissected back to the aorta and inferior vena cava (IVC) of twin A, and ultimately anastamosed end-to-side to the aorta and IVC of twin B. The sciatic nerve of the donor limb received contributions from both twins, and the portion from twin B was preserved. The remaining portion of sciatic nerve from twin A was connected to the sciatic stump of twin B. Finally, the previously isolated anterior thigh muscles (still perfused and innervated from the recipient) were connected to the quadriceps tendon of the donor limb. Additional procedures included femoral osteotomies and quadriceps tendon shortening at 2 y of age to correct abduction and weak knee extension, and semitendinosus and biceps femoris tendon transfers to augment knee extension at 6 y of age.
At 6 y of age, the transplanted limb was 6.5cm shorter than the native limb, likely due to length discrepancies at time of transplant. Sensation to light touch is present but diminished throughout the entire limb, with the greatest difference in the upper thigh; none of which causes functional impairment. Passive range of motion is near normal throughout, although active range of motion is markedly decreased. Hip flexion, knee extension/flexion, and the great toe demonstrate good strength (grade 3-4). The patient lacks power in ankle plantar- and dorsiflexion. Most importantly, with bracing, the patient is able to ambulate and participate in recreational activities with her peers. Functional MRI shows cortical integration of the limb.
Case 2
Cavadas et al. performed the first adult lower extremity allotransplantation in July, 2011.Citation7,26 Bilateral trans-femoral transplantations were performed in a 22 year-old male recipient. The recipient had high bilateral traumatic above knee amputations (AKA) sustained 2 y prior to transplantation following a motor vehicle collision (). Pain and socket instability prevented the use of conventional prostheses and the patient refused osseointegrated prostheses due to poor experiences of peers with lower extremity amputations. The donor was a 26 year-old female multi-organ donor. The pairing was a full HLA mismatch with both donor and recipient being Cytomegalovirus (CMV) seronegative. The recipient was Epstein-Barr virus (EBV) IgG positive and the donor was EBV IgG negative.
Donor lower extremities were procured with vasculature at the external iliac vessels, sciatic nerve at the sciatic notch, all muscles of the anterior and posterior thighs included, and osteotomies at 22 and 12 cm proximal to the knee on the right and left sides, respectively. Osteotomy levels were based on the recipient's previous height and preoperative imaging. The entire body was cold perfused in-situ with University of Wisconsin (UW) solution without cross clamping. Heart and liver were procured first. The right leg was then procured and immediately transferred to the recipient's operating room for transplantation without further cooling. The left leg was procured and kept cool on ice while the right leg was transplanted. Donor limbs were perfused with warm Ringer's solution immediately prior to revascularization to help prevent hypothermia. Both lower extremities were repaired in the same sequence: osteosynthesis with 4.5mm locking plates, sciatic nerve epineural coaptation, muscle repair, and end-to-side external iliac anastamosis. Ischemia times were 3.5 and 5.5 hours for right and left lower extremities, respectively. Both legs were transplanted at the distal femoral level, though a more proximal level of the distal femoral zone on the right.
The patient was induced with alemtuzumab (anti-CD52 monoclonal antibody) and maintained on a tripe-drug immunosuppressive regimen consisting of tacrolimus, mycophenolate mophetyl (MMF), and steroid taper. He incurred 2 Banff grade I acute rejection episodes in the first year, one related to acute CMV infection and one related to immunosuppression dose reductions. Two abscesses developed in the quadriceps, thought to be related to distal muscle ischemia, treated with debridement and tailored intravenous antibiotics. Ultrasound based bone stimulation was used for left femoral non-union.
At one-year follow-up, the recipient was ambulating with partial weight bearing on parallel bars. He had full passive range of motion of both knees, with 45° and 30° active extension lags in the right and left legs, respectively. Plantar flexion was 3+ and 4 (MRC score) in the right and left feet, respectively. Tinel's sign had advanced to the plantar level. Sensation and proprioception were not reported. Unfortunately, due to development of brain lymphoma, immunosuppression was ceased, resulting in rejection and removal of both limbs.Citation27,28
Case 3
Ozkan and colleagues performed the world's first triple limb transplant in Turkey in 2012.Citation1,8,Citation29 Unfortunately, there are no literature reports of the operation, and the only details available are from media accounts. The recipient was a 34 year-old man who lost both arms and his right leg from an electrocution injury as a child (). The donor was a man who was declared brain dead after being hit by a train. The operation lasted 12 hours. By postoperative day one, the transplanted right leg was removed due to what was reported as "rejection." The patient was discharged on postoperative day 69, but ultimately died within 5 months from infectious complications.Citation21
Case 4
A team led by Erdem reported the world's first attempted quadruple extremity allotransplantation, which was also performed in Turkey.Citation22-24 The procedure was carried out in February 2012. The recipient was a 27 year-old man who lost all 4 extremities from an electrical accident at the age of 13 y (). He had a left trans-humeral, right shoulder level, and bilateral proximal trans-femoral amputations. The donor was a 40 year-old man with a full HLA mismatch.
All four extremities, as well as the donor's face (donated to a separate recipient), were harvested simultaneously with the solid organ transplant teams. The limbs were perfused with UW solution. The team's major goal was to reduce ischemia time. To accomplish their primary goal, 3 microsurgical teams worked simultaneously to revascularize the extremities, and the lower extremities were connected via temporary shunts while the upper extremities were definitively reperfused. Due to the high level amputations, the team had difficulty maintaining access for both resuscitation and blood pressure monitoring. Intra-operative metabolic derangements evolved, eventually leading to hyperkalemia, bradycardia, and cardiac arrest at the completion of the last anastamosis. Sternotomy and cardiopulmonary support was required to reestablish spontaneous circulation. Continuous hemodyalisis and massive transfusion (200 units) ensued over the immediate postoperative period. Each limb was sequentially amputated. The patient died on the fourth postoperative day.
Immunosuppression consisted of anti-thymocyte globulin (ATG) induction and triple-drug maintenance therapy with tacrolimus, MMF, and corticosteroids. The donor extremities were irradiated due to unclear evidence of benefit in a small animal model performed at their hospital.Citation30
The report of this case is limited and many questions are unanswered. Details of preoperative planning are incomplete. Due to the descriptions of the recipient vasculature intraoperatively, it appears there was no preoperative recipient vascular imaging. Exact management of the perfusion of the limbs prior to anastamosis is vague. No mention of preemptive transfusion for the anticipated increased blood volume distribution corresponding to the added intravascular space was mentioned.
Limitations of Lower Extremity Prosthetics
Good functional outcome can be achieved in most patients with lower extremity amputations, but is largely dependent upon the level of the amputation. There is a progressive and significant lowering of physical component score (worsening outcomes) as unilateral amputation level becomes more proximal from below knee to through knee to above knee.Citation16 Energy expenditure in ambulation increases with higher levels of amputation, with up to 200% increase above normal levels in patients with bilateral trans-femoral amputations.Citation17 Decreased quality of life can be expected in patients with more proximal levels of lower extremity amputations. Hagberg and colleagues found that the most frequently reported problems that led to reduction in quality of life were heat/sweating in the prosthetic socket (72%), sores/skin irritation from the socket (62%), inability to walk in woods and fields (61%), and inability to walk quickly (59%).Citation18 Above knee amputation is a statistically significant independent risk factor for an amputee to not wear a prosthesis.Citation19
In an effort to alleviate problems with conventional prosthetics in trans-femoral amputees, percutaneous osseointegrated prostheses were introduced in Sweden in the 1990s.Citation31 Implant survival was 92% after 2 years, with improved daily prosthetic use, prosthetic mobility, global situation, physical function, and fewer problems. However, patients with osseointegrated prostheses commonly experience superficial infections (>50%).Citation32
Myoelectric prosthetics offer a high degree of freedom of movement using robotic technology coupled to electromyographic signals generated over target muscles.Citation33 The limiting step in applying myoelectric prosthetics more widely is harnessing specific, accurate, and long-lasting input. First generation myoelectric prosthetics were constrained by the available functioning muscle groups, with proximal upper extremity amputees having limited input (i.e. biceps and triceps). Targeted muscle reinnervation (TMR) is one strategy to provide increased inputs by transferring available nerves to power available muscles, with native nerves providing intuitive functions to the robotic prosthetic (i.e., radial nerve extends wrist and fingers). However, TMR is still limited by available nerves, available amplifiers (muscles), and inconsistent surface electrodes.Citation33-39 Alternative solutions include intracranial electrode placement for brain controlCitation40-45 and highly selective peripheral nerve interfaces.Citation33,46-48
Challenges Facing Lower Extremity Transplantation
Nerve regeneration
One of the greatest challenges for lower extremity allotransplantation is the slow pace of nerve regeneration and functional recovery. As with upper extremity allotransplantation, the best recovery and outcome can be expected for more distal transplants; however, these patients have better function with prosthetics. Nerve regeneration is classically described as proceeding at 1 mm/day, and potentially faster in the presence of tacrolimus.Citation49-57 Cavadas et al. reported an advancing Tinel's sign of 2.5 mm/day in the patient that received bilateral lower extremity allotransplanations.Citation7 With lower extremity lengths between 70-100cm, anticipated times for nerve regeneration of an entire lower limb vary greatly (280-1,000 days). In cases of nerve injury with reconstruction, gains in function are rarely seen beyond one year. However, upper extremity allotransplantation patients have acquired increased motor and sensory action potentials up to 5 y post-transplantation.Citation49 Within the first year, regeneration is required to prevent distal Schwann cells from becoming quiescent and degeneration of neuromuscular junctions, resulting in muscle atrophy and fibrosis.Citation51 Successful strategies for improved nerve regeneration would greatly influence the risk-benefit ratio of lower extremity allotransplantation, especially cases of proximal transplantation. Taking advantage of the neuroregenerative properties of tacrolimus, fully supporting or replacing Schwann cells, tailoring surgical technique to preserve motor/sensory fascicle anatomy, and decompressing known locations of peripheral nerve entrapment are a few approaches to optimize nerve regeneration in VCA.Citation49,51
In contrast to upper extremity allotransplantation, lower extremity transplants do not require significant functional recovery beyond the extrinsic muscles of the foot. Ankle dorsi/plantar flexion and great toe extension/flexion can be achieved with muscles of the anterior and posterior compartments of the lower leg. In addition, leg transplantation only requires protective plantar sensation, as opposed to the goal of discriminatory sensibility in hand transplantation. Ninety percent of radiocarpal/distal forearm level upper extremity transplant recipients achieved protective sensation within the first post-transplant year, and cortical reintegration has been consistently demonstrated in VCA.Citation50,58-60
Blood volume re-distribution
The lower extremity comprises more than 3 times the percentage of total body mass when compared to the upper extremity (17-18% vs. 5-6%), depending on gender.Citation61 Traditional Lund-Browder charts estimate the lower extremities to have twice the total body surface area compared to upper extremities (18% vs 9%, respectively).Citation62 Each lower extremity comprises approximately 10% of the total body intravascular volume depending on body positioning and vascular tone.Citation63-65 Taken together, fluid and blood volume status must be carefully considered prior to lower extremity transplantation; more so than in upper extremity allotransplantation. Preservation fluid in the donor limb will create a bolus to the rest of the body upon reperfusion. Exchange of cold preservation fluid for fresh, warm intravenous fluid immediately prior to revascularization may be preferable, decreasing hypothermia and potentially improving metabolic imbalances. We have previously advocated for preemptive blood transfusion to prevent coagulopathy in upper extremity transplantation, which was done in the case by Cavadas et al. for lower extremity transplantation, ensuring adequate blood volume to fill the newly added intravascular space.Citation66 Bilateral lower extremity transplantation complicates the picture further, and timing of reperfusion of donor limbs is critical. Case 4 highlights this complexity of adequate resuscitation and metabolic management. These issues become less of a concern when discussing more distal transplantations, as the thigh is disproportionately larger than the leg.
Lifelong immunosuppression
As with all forms of allotransplantation, the risk of lifelong immunosuppression must be carefully weighed against the benefits of the procedure. Although experimental and clinical research is making strides toward reducing the requirements for immunosuppression, or even inducing tolerance, the current reality of immunosuppression remains.Citation67-72 In patients with multiple previous reconstructive procedures, requiring blood transfusions and allogeneic skin grafts, the presence of donor-specific antibodies (DSA) is a threat to graft stability in reconstructive transplantation. Further research is underway to explore the full role of DSA in VCA, and is another obstacle to overcome in the path to tolerance.Citation73 The prospect of nephrotoxicity, hepatotoxicity, infection, and neoplastic growth are real concerns. Compliance with immunosuppression is critical to achieving long-term success and has proved to be one of the greatest obstacles in selecting the correct patients. The initial recipients of lower extremity allotransplantation need to be selected carefully based on lessons learned from other VCA recipients.
Benefits of Lower Extremity Transplantation
Given the limitations of current and developing prosthetics, lower extremity allotransplantation offers unique benefits. With nearly all upper extremity allotransplantation recipients achieving protective sensation,Citation50 it is possible that similar outcomes are possible with lower extremity transplantation. Discriminatory sensation would be an added benefit, but not a goal as it is with hand transplantation. Therefore, "functional" sensory recovery in lower extremity allotransplantation is expected over time. Blume et al. found that pain in replanted upper extremities was inversely correlated with the amount of cortical reorganization.Citation74 With a high degree of cortical plasticity observed in upper extremity allotransplantation recipients and the first case of leg transplantation by Zuker and colleagues, lower extremity allotransplantation may reduce pain and phantom limb sensations.Citation5,50,Citation58-60 The prospect of restoring protective sensation and alleviating phantom limb phenomena not only provides benefits beyond current prosthetics, but could allow lower extremity amputees to feel whole again. If patients are adequately screened and selected, and are able to develop mature coping mechanisms, lower extremity transplantation may improve their image and self concept compared to the constant reminder of a lost limb induced by the presence of a prosthetic.Citation75
Permanence provided by extremity transplantation avoids daily problems and decisions experienced with prosthetics. Poor fitting prostheses result in pressure sores and instability, particularly in high-level amputees. Skin irritation, socket heat, and the inability to walk quickly or on uneven terrain result in decreased prosthetic use.Citation18,19 Lower extremity allotransplantation will alleviate socket-fitting issues and eliminate the choice of wearing the prosthetic. In addition, performing a lower extremity transplantation to extend an above-knee amputation to a below-knee level amputation (i.e. not transplanting the foot) would provide a stable distal stump for a prosthesis socket, improve functional ambulation, and decrease the work of ambulation. Extending the level of amputation distally could greatly improve quality of life. Such a procedure could potentially be combined with distal hand or face transplantation. The expansion of intra-vascular volume by amputation level extension would not be affected to the same degree as in proximal femoral transplantation utilizing the entire lower limb. Combining amputation level extension with face or distal upper extremity transplantation may reduce complications related to increased intravascular space and large volume ischemic tissue, when compared to combination with proximal level extremity transplantation. Lower extremity amputation level extension would require little motor nerve regeneration, if any, as the quadriceps are typically preserved in patients with above knee amputations.
Figure 2. Classification of lower extremity transplantation levels base on preserved recipient muscle innervation: (A) proximal femur with no retained innervation, (B) mid-femur with preserved quadriceps, (C) distal femur with preserved anterior and posterior thigh muscle innervation, but above the knee, (D) proximal tibia is below the knee, but without innervation of any lower leg muscles, (E), mid-tibia is based off typical stair-stepped below knee amputation incisions, with preserved posterior compartment muscles but no anterior compartment innervation, and (F) distal tibia with innervation to all extrinsic muscles of the foot retained.
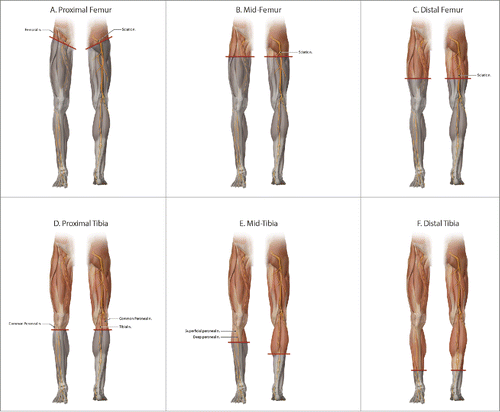
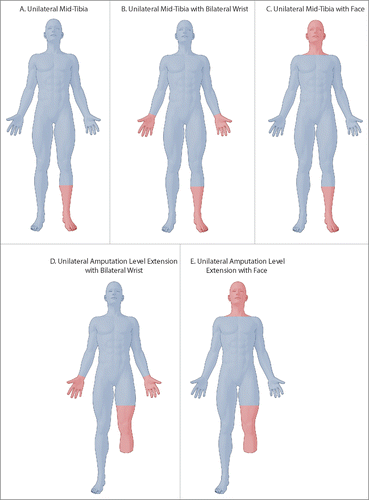
Figure 3. Illustrations of proposed next lower extremity allotransplantation recipient: (A) unilateral mid-tibia, (B) unilateral mid-tibia with bilateral wrist, (C) unilateral mid-tibia with face, (D) unilateral amputation level extension with bilateral wrist, and (E) unilateral amputation level extension with face. Note distal level of proposed hand transplants in B and D. Blue highlights recipient's preoperative status and red highlights allotransplants.
Overall, lower extremity allotransplantation has the potential to offer patients improved function, restoration of self, reduction in pain, and avoidance of prosthetic associated complications. Well-conceived strategies are required to optimize outcomes and gain knowledge in this experimental field.
Lower Extremity Allotransplantation Classification
Before lower extremity allotransplantation cases accumulate, it is wise to establish a uniform categorization for consistent reporting and analysis of results. Lower extremity allotransplantation can be divided into levels based on retained recipient motor innervation of the various muscle groups: proximal femur, mid-femur, distal femur, proximal tibia, mid-tibia, and distal tibia (). In proximal femoral transplantation, no native muscle innervation remains, femoral and sciatic nerves are repaired, and must regenerate the length of the limb to regain function of donor muscles (). In mid-femoral transplantation, native quadriceps innervation is preserved, but sciatic repair and regeneration is required to innervate the hamstrings and leg musculature (). Distal femoral transplantation has intact anterior and posterior thigh muscle compartments, but remains above the knee and the sciatic nerve is coapted distally to reinnervate the leg (). Proximal tibial transplantation is below the knee, but again no native innervation of the anterior and posterior leg compartments is preserved (). At this level, the tibial and common peroneal nerves are repaired. In terms of below knee transplantation, the most common scenario is likely a mid-tibial level (). The typical incision and flap design for below knee amputation is the stair-step incision, with a proximal anterior incision and a more distal posterior incision. This creates a soft tissue flap composed of the gastrocnemius and soleus muscles to cover the exposed bone. Due to this common amputation approach, a mid-tibial transplantation has retained posterior leg compartment innervation, but denervated anterior leg compartment muscles. Superficial and deep peroneal nerves are repaired to restore lateral and anterior compartment motor function, respectively. The tibial nerve is coapted for reinnervation of the deep posterior compartment muscles. A patient presenting with a distal tibial amputation level is rare, as most are revised to a mid-tibial level for prosthetic fit and function. For distal tibial transplantation, all extrinsic muscles to the foot are preserved in the recipient, and nerve repair is strictly for sensory recovery and incremental gains in intrinsic foot musculature (). The best functional outcome can be expected at the distal tibia, similar to distal forearm level upper extremity allotransplantation. Even with preserved recipient muscle innervation, further procedures may be required to optimize function, including tendon length adjustments and tendon transfers, as performed by Zuker et al.Citation5,6
Current Indications For Lower Extremity Transplantation
As various centers are moving forward with lower extremity allotransplantation, it is important to proceed in a stepwise and safe manner. The first case by Zuker et al. was a model case for the field, with a high benefit-to-risk ratio due to no immunosuppression requirement in a patient that was undergoing a major operation regardless of the transplant, and had a relatively high potential for nerve regeneration due to age and nerve length to end-organs of innervation. However, with each new case performed, there have been large leaps, resulting in mortalities in the latter 2 cases. These results emphasize the need for proper recipient selection and an exquisitely detailed and well-rehearsed operative plan. Proximal levels of amputation necessitate intricate preparation for staged anastomosis, resuscitation, reperfusion sequence, and critical care. Combining high-level upper extremity transplantations with high-level lower extremity transplantations may be ill conceived at this early stage without more clinical success in less complex cases. More knowledge is needed concerning the optimal pre-, intra-, and postoperative management of high-level lower extremity transplantations alone.
We believe that it is best to proceed with distal lower extremity allotransplantations first, with the ability to learn about functional recovery without needing to manage a highly complex blood volume re-distribution, large masses of skeletal muscle undergoing ischemia-reperfusion injury, and large antigenic loads (). Only by observing the functional recovery of distal level transplants, will we be able to decide if the benefit of proximal level transplantation is worth the risk of perioperative complications combined with lifelong imunnosuppression. A practical recipient for early stage lower extremity allotransplantation would be a patient requiring a distal forearm level upper extremity transplant or face transplantation combined with a unilateral distal tibial transplant (). Although there have been poor outcomes with combined VCA cases,Citation8,21-24,Citation76 selectively choosing multiple distal transplants could mitigate concerns regarding massive blood volume shifts, high antigenic burden, and large tissue mass ischemia. Futhermore, we only endorse combined VCA cases to be attempted by teams that are experienced with distal forearm or face transplantation in isolation before attempting concomitant lower extremity transplantation.
Along the same lines, opportunity exists to extend amputation level from above knee to below knee for improved prosthetic function. This would allow for analysis of thigh muscle functional recovery without requiring complete lower extremity transplantation. Amputation level extension would ideally be performed in a hand transplant candidate that is a triple or quadruple limb amputee (). Providing such a patient with hand function and extending the level of lower extremity amputation would significantly improve prosthetic use and hopefully the ability to ambulate. Amputation level extension could similarly be combined with face transplantation (). Again, teams should be intricately familiar with hand or face transplantation before combining with lower extremity amputation level extension.
These two scenarios (distal tibia with distal forearm transplant, or trans-femoral to trans-tibial amputation level extension with distal forearm transplant) may be difficult to identify, but would provide definitive answers about functional recovery with minimal added risk. It is not currently clear that proximal level lower extremity transplants are indicated by themselves, and it has been demonstrated that combining proximal level lower extremity transplants with upper extremity transplants results in a high likelihood of death. Given this, we endorse/advocate for taking small first steps as was done in isolated upper extremity and isolated face allotransplantation. We foresee the field ultimately proceeding to performing transplants similar to those by Cavdas et al. (bilateral mid-femur), but think it is wise to be selective in the near future to ensure success, safety, and to gain incremental knowledge.
Conclusions
Lower extremity allotransplantation offers patients the possibility of improved function, restoration of self, reduction in pain, and avoidance of prosthetic associated complications. Improvements in nerve regeneration, fluid resuscitation, and immunotherapy would favor further expansion into lower extremity transplantation. Lower extremity allotransplantation can be classified according to the degree of preservation of recipient muscle innervation: proximal femur, mid-femur, distal femur, proximal tibia, mid-tibia, and distal tibia. The next cases should optimize outcomes by performing distal level transplantations or amputation level extensions in order to gain knowledge of functional recovery while minimizing perioperative morbidity and mortality.
Disclosure of Potential Conflicts of Interest
The authors have no personal or financial conflicts of interest related to this subject matter or manuscript to disclose. No funding was used in preparation of this manuscript.
Acknowledgments
The authors would like to acknowledge David Blum, MA, PhD, of BlumDesignWerks for medical illustrations in Figures 1c, 2, and 3.
References
- Shores JT, Brandacher G, Lee WA. Hand and Upper Extremity Transplantation: An Update of Outcomes in the Worldwide Experience. Plast Reconstr Surg 2015; 135(2):351e–60e
- Khalifian S, Brazio PS, Mohan R, Shaffer C, Brandacher G, Barth RN, Rodriguez ED. Facial transplantation: the first 9 years. Lancet 2014; 384:2153-63; PMID:24783986; http://dx.doi.org/10.1016/S0140-6736(13)62632-X
- Petruzzo P, Dubernard JM. The International Registry on Hand and Composite Tissue allotransplantation. Clin Transpl 2011:247-53; PMID:22755418
- Petruzzo P, Gazarian A, Kanitakis J, Parmentier H, Guigal V, Guillot M, Vial C, Dubernard JM, Morelon E, Badet L. Outcomes after bilateral hand allotransplantation: a risk/benefit ratio analysis. Ann Surg 2015; 261:213-20; PMID:24646555; http://dx.doi.org/10.1097/SLA.0000000000000627
- Fattah A, Cypel T, Donner EJ, Wang F, Alman BA, Zuker RM. The first successful lower extremity transplantation: 6-year follow-up and implications for cortical plasticity. Am J Transplant 2011; 11:2762-7; PMID:21991888; http://dx.doi.org/10.1111/j.1600-6143.2011.03782.x
- Zuker RM, Redett R, Alman B, Coles JG, Timoney N, Ein SH. First successful lower-extremity transplantation: technique and functional result. J Reconstr Microsurg 2006; 22:239-44; PMID:16783680; http://dx.doi.org/10.1055/s-2006-939928
- Cavadas PC, Thione A, Carballeira A, Blanes M. Bilateral transfemoral lower extremity transplantation: result at 1 year. Am J Transplant 2013; 13:1343-9; PMID:23433015; http://dx.doi.org/10.1111/ajt.12178
- Turkish hospital performs triple limb and face transplant. Today's Zaman. 2012. Retrieved from http://www.todayszaman.com/news-269227-turkish-hospital-performs-triple-limb-and-face-transplant.html
- Berli JU, Broyles JM, Lough D, Shridharani SM, Rochlin D, Cooney DS, Lee WP, Brandacher G, Sacks JM. Current concepts and systematic review of vascularized composite allotransplantation of the abdominal wall. Clin Transplant 2013; 27:781-9; PMID:24102820; http://dx.doi.org/10.1111/ctr.12243
- Broyles JM, Berli J, Tuffaha SH, Sarhane KA, Cooney DS, Eckhauser FE, Lee WP, Brandacher G, Singh DP, Sacks JM. Functional Abdominal Wall Reconstruction Using an Innervated Abdominal Wall Vascularized Composite Tissue Allograft: A Cadaveric Study and Review of the Literature. J Reconstr Microsurg 2015; 31(1):39–4; PMID:25184615
- Tuffaha SH, Sacks JM, Shores JT, Brandacher G, Lee WP, Cooney DS, Redett RJ. Using the dorsal, cavernosal, and external pudendal arteries for penile transplantation: technical considerations and perfusion territories. Plast Reconstr Surg 2014; 134:111e-9e; PMID:24622570; http://dx.doi.org/10.1097/PRS.0000000000000277
- Carty MJ, Zuker R, Cavadas P, Pribaz JJ, Talbot SG, Pomahac B. The case for lower extremity allotransplantation. Plast Reconstr Surg 2013; 131:1272-7; PMID:23416434; http://dx.doi.org/10.1097/PRS.0b013e31828bd1a5
- Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil 2008; 89:422-9; PMID:18295618; http://dx.doi.org/10.1016/j.apmr.2007.11.005
- Fischer H. A Guide to US. Military Caualty Statistics: Operation New Dawn, Operation Iraqi Freedom, and Operation Enduring Freedom. Congress Research Service 2014.
- Carty MJ, Duclos A, Talbot SG, Tullius SG, Pribaz JJ, Pomahac B. Attitudes regarding lower extremity allotransplantation among lower extremity amputees. Plast Reconstr Surg 2014; 134:1334-42; PMID:25255108; http://dx.doi.org/10.1097/PRS.0000000000000658
- Penn-Barwell JG. Outcomes in lower limb amputation following trauma: a systematic review and meta-analysis. Injury 2011; 42:1474-9; PMID:21831371; http://dx.doi.org/10.1016/j.injury.2011.07.005
- Cuccurullo SJ. Physical medicine and rehabilitation board review. New York: Demos Medical Publishing, 2004.
- Hagberg K, Branemark R. Consequences of non-vascular trans-femoral amputation: a survey of quality of life, prosthetic use and problems. Prosthet Orthot Int 2001; 25:186-94; PMID:11860092; http://dx.doi.org/10.1080/03093640108726601
- Taylor SM, Kalbaugh CA, Blackhurst DW, Hamontree SE, Cull DL, Messich HS, Robertson RT, Langan EM, 3rd, York JW, Carsten CG, 3rd, et al. Preoperative clinical factors predict postoperative functional outcomes after major lower limb amputation: an analysis of 553 consecutive patients. J Vasc Surg 2005; 42:227-35; PMID:16102618; http://dx.doi.org/10.1016/j.jvs.2005.04.015
- Alexander RM. Bipedal animals, and their differences from humans. J Anat 2004; 204:321-30; PMID:15198697; http://dx.doi.org/10.1111/j.0021-8782.2004.00289.x
- Sheets CA. Atilla Kavdir dead months after receiving triple-limb transplant in Turkey. International Business Times. 2012. Retrieved from http://www.ibtimes.com/atilla-kavdir-dead-months-after-receiving-triple-limb-transplant-turkey-694734
- Nasir S, Kilic YA, Karaaltin MV, Erdem Y. Lessons learned from the first quadruple extremity transplantation in the world. Ann Plast Surg 2014; 73:336-40; PMID:25121416
- Landin L, Dominguez A, Sanchez-Sanchez M. Discussion of lessons learned from the first quadruple extremity transplantation in the world: the logic of massive allograft transplantation. Ann Plast Surg 2014; 73:341-2; PMID:25121417; http://dx.doi.org/10.1097/SAP.0000000000000280
- Swanson EW, Brandacher G, Gordon CR. Discussion of lessons learned from the first quadruple extremity transplantation in the world: comments and concerns regarding quadruple extremity allotransplantation. Ann Plast Surg 2014; 73:343-5; PMID:25003409; http://dx.doi.org/10.1097/SAP.0000000000000281
- Merrill JP, Murray JE, Harrison JH, Guild WR. Successful homotransplantation of the human kidney between identical twins. J Am Med Assoc 1956; 160:277-82; PMID:13278189; http://dx.doi.org/10.1001/jama.1956.02960390027008
- Vicente D, Potter BK, Elster E. "Just because you can, does not mean that you should." Am J Transplant 2013; 13:1123-4; PMID:23480206; http://dx.doi.org/10.1111/ajt.12176
- American Society for Reconstructive Transplantation 3rd Biennial Meeting. Chicago, IL, 2012.
- First double leg-transplant patient has legs amputated. BBC News Health, 2013.
- Shores JT, Lee WP, Brandacher G. Discussion: Lessons learned from simultaneous face and bilateral hand allotransplantation. Plast Reconstr Surg 2013; 132:433-4; PMID:23897340; http://dx.doi.org/10.1097/PRS.0b013e31829588eb
- Yavas G, Yildiz F, Guler S, Sargon MF, Yildiz D, Yolcu T, Tuncer M, Akyol FH. Concomitant trastuzumab with thoracic radiotherapy: a morphological and functional study. Ann Oncol 2011; 22:1120-6; PMID:21097554; http://dx.doi.org/10.1093/annonc/mdq590
- Branemark R, Branemark PI, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev 2001; 38:175-81; PMID:11392650
- Branemark R, Berlin O, Hagberg K, Bergh P, Gunterberg B, Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: A prospective study of 51 patients. Bone Joint J 2014; 96-B:106-13; PMID:24395320; http://dx.doi.org/10.1302/0301-620X.96B1.31905
- Kung TA, Bueno RA, Alkhalefah GK, Langhals NB, Urbanchek MG, Cederna PS. Innovations in prosthetic interfaces for the upper extremity. Plast Reconstr Surg 2013; 132:1515-23; PMID:24281580; http://dx.doi.org/10.1097/PRS.0b013e3182a97e5f
- Tenore FV, Ramos A, Fahmy A, Acharya S, Etienne-Cummings R, Thakor NV. Decoding of individuated finger movements using surface electromyography. IEEE Trans Biomed Eng 2009; 56:1427-34; PMID:19473933; http://dx.doi.org/10.1109/TBME.2008.2005485
- Dumanian GA, Ko JH, O'Shaughnessy KD, Kim PS, Wilson CJ, Kuiken TA. Targeted reinnervation for transhumeral amputees: current surgical technique and update on results. Plast Reconstr Surg 2009; 124:863-9; PMID:19730305; http://dx.doi.org/10.1097/PRS.0b013e3181b038c9
- Kuiken TA, Marasco PD, Lock BA, Harden RN, Dewald JP. Redirection of cutaneous sensation from the hand to the chest skin of human amputees with targeted reinnervation. Proc Natl Acad Sci U S A 2007; 104:20061-6; PMID:18048339; http://dx.doi.org/10.1073/pnas.0706525104
- Kuiken TA, Miller LA, Lipschutz RD, Lock BA, Stubblefield K, Marasco PD, Zhou P, Dumanian GA. Targeted reinnervation for enhanced prosthetic arm function in a woman with a proximal amputation: a case study. Lancet 2007; 369:371-80; PMID:17276777; http://dx.doi.org/10.1016/S0140-6736(07)60193-7
- O'Shaughnessy KD, Dumanian GA, Lipschutz RD, Miller LA, Stubblefield K, Kuiken TA. Targeted reinnervation to improve prosthesis control in transhumeral amputees. A report of three cases. J Bone Joint Surg Am 2008; 90:393-400; PMID:18245601; http://dx.doi.org/10.2106/JBJS.G.00268
- Ohnishi K, Weir RF, Kuiken TA. Neural machine interfaces for controlling multifunctional powered upper-limb prostheses. Expert Rev Med Devices 2007; 4:43-53; PMID:17187470; http://dx.doi.org/10.1586/17434440.4.1.43
- Shih JJ, Krusienski DJ. Signals from intraventricular depth electrodes can control a brain-computer interface. J Neurosci Methods 2012; 203:311-4; PMID:22044847; http://dx.doi.org/10.1016/j.jneumeth.2011.10.012
- Shih JJ, Krusienski DJ, Wolpaw JR. Brain-computer interfaces in medicine. Mayo Clin Proc 2012; 87:268-79; PMID:22325364; http://dx.doi.org/10.1016/j.mayocp.2011.12.008
- McFarland DJ, Sarnacki WA, Wolpaw JR. Electroencephalographic (EEG) control of three-dimensional movement. J Neural Eng 2010; 7:036007; PMID:20460690; http://dx.doi.org/10.1088/1741-2560/7/3/036007
- Yanagisawa T, Hirata M, Saitoh Y, Goto T, Kishima H, Fukuma R, Yokoi H, Kamitani Y, Yoshimine T. Real-time control of a prosthetic hand using human electrocorticography signals. J Neurosurg 2011; 114:1715-22; PMID:21314273; http://dx.doi.org/10.3171/2011.1.JNS101421
- Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP. Instant neural control of a movement signal. Nature 2002; 416:141-2; PMID:11894084; http://dx.doi.org/10.1038/416141a
- Schultz AE, Kuiken TA. Neural interfaces for control of upper limb prostheses: the state of the art and future possibilities. PM R 2011; 3:55-67; PMID:21257135; http://dx.doi.org/10.1016/j.pmrj.2010.06.016
- Navarro X, Krueger TB, Lago N, Micera S, Stieglitz T, Dario P. A critical review of interfaces with the peripheral nervous system for the control of neuroprostheses and hybrid bionic systems. J Peripher Nerv Syst 2005; 10:229-58; PMID:16221284; http://dx.doi.org/10.1111/j.1085-9489.2005.10303.x
- Chu JU, Song KI, Han S, Lee SH, Kim J, Kang JY, Hwang D, Suh JK, Choi K, Youn I. Improvement of signal-to-interference ratio and signal-to-noise ratio in nerve cuff electrode systems. Physiol Meas 2012; 33:943-67; PMID:22551721; http://dx.doi.org/10.1088/0967-3334/33/6/943
- Urbanchek MG, Wei B, Egeland BM, Abidian MR, Kipke DR, Cederna PS. Microscale electrode implantation during nerve repair: effects on nerve morphology, electromyography, and recovery of muscle contractile function. Plast Reconstr Surg 2011; 128:270e-8e; PMID:21921739; http://dx.doi.org/10.1097/PRS.0b013e3182268ac8
- Brandacher G, Gorantla VS, Lee WP. Hand allotransplantation. Semin Plast Surg 2010; 24:11-7; PMID:21286301; http://dx.doi.org/10.1055/s-0030-1253243
- Shores JT, Imbriglia JE, Lee WP. The current state of hand transplantation. J Hand Surg Am 2011; 36:1862-7; PMID:22036285; http://dx.doi.org/10.1016/j.jhsa.2011.09.001
- Glaus SW, Johnson PJ, Mackinnon SE. Clinical strategies to enhance nerve regeneration in composite tissue allotransplantation. Hand Clin 2011; 27:495-509, ix; PMID:22051390; http://dx.doi.org/10.1016/j.hcl.2011.07.002
- Doolabh VB, Mackinnon SE. FK506 accelerates functional recovery following nerve grafting in a rat model. Plast Reconstr Surg 1999; 103:1928-36; PMID:10359255; http://dx.doi.org/10.1097/00006534-199906000-00018
- Jost SC, Doolabh VB, Mackinnon SE, Lee M, Hunter D. Acceleration of peripheral nerve regeneration following FK506 administration. Restor Neurol Neurosci 2000; 17:39-44; PMID:11490076
- Lee M, Doolabh VB, Mackinnon SE, Jost S. FK506 promotes functional recovery in crushed rat sciatic nerve. Muscle Nerve 2000; 23:633-40; PMID:10716776; http://dx.doi.org/10.1002/(SICI)1097-4598(200004)23:4%3c633::AID-MUS24%3e3.0.CO;2-Q
- Gold BG, Katoh K, Storm-Dickerson T. The immunosuppressant FK506 increases the rate of axonal regeneration in rat sciatic nerve. J Neurosci 1995; 15:7509-16; PMID:7472502
- Sulaiman OA, Voda J, Gold BG, Gordon T. FK506 increases peripheral nerve regeneration after chronic axotomy but not after chronic schwann cell denervation. Exp Neurol 2002; 175:127-37; PMID:12009765; http://dx.doi.org/10.1006/exnr.2002.7878
- Udina E, Ceballos D, Gold BG, Navarro X. FK506 enhances reinnervation by regeneration and by collateral sprouting of peripheral nerve fibers. Exp Neurol 2003; 183:220-31; PMID:12957505; http://dx.doi.org/10.1016/S0014-4886(03)00173-0
- Vargas CD, Aballea A, Rodrigues EC, Reilly KT, Mercier C, Petruzzo P, Dubernard JM, Sirigu A. Re-emergence of hand-muscle representations in human motor cortex after hand allograft. Proc Natl Acad Sci U S A 2009; 106:7197-202; PMID:19366678; http://dx.doi.org/10.1073/pnas.0809614106
- Dubernard JM, Petruzzo P, Lanzetta M, Parmentier H, Martin X, Dawahra M, Hakim NS, Owen E. Functional results of the first human double-hand transplantation. Ann Surg 2003; 238:128-36; PMID:12832975
- Frey SH, Bogdanov S, Smith JC, Watrous S, Breidenbach WC. Chronically deafferented sensory cortex recovers a grossly typical organization after allogenic hand transplantation. Curr Biol 2008; 18:1530-4; PMID:18848443; http://dx.doi.org/10.1016/j.cub.2008.08.051
- Plagenhoef S, Evans FG, Abdelnour T. Anatomical data for analyzing human motion. Res Q Exerc Sport 1983; 54:169-78; http://dx.doi.org/10.1080/02701367.1983.10605290
- Malic CC, Karoo RO, Austin O, Phipps A. Resuscitation burn card–a useful tool for burn injury assessment. Burns 2007; 33:195-9; PMID:17222978; http://dx.doi.org/10.1016/j.burns.2006.07.019
- Litter J, Wood JE. The volume and distribution of blood in the human leg measured in vivo. I. The effects of graded external pressure. J Clin Invest 1954; 33:798-806; PMID:13163171; http://dx.doi.org/10.1172/JCI102951
- Asmussen E. The distribution of the blood between the lower extremities and the rest of the body. Acta Physiol Scandinav 1943; 5:31; http://dx.doi.org/10.1111/j.1748-1716.1943.tb02030.x
- Ebert RV, Stead EA. The Effect of the Application of Tourniquets on the Hemodynamics of the Circulation. J Clin Invest 1940; 19:561-7; PMID:16694773; http://dx.doi.org/10.1172/JCI101159
- Lang RS, Gorantla VS, Esper S, Montoya M, Losee JE, Hilmi IA, Sakai T, Lee WP, Raval JS, Kiss JE, et al. Anesthetic management in upper extremity transplantation: the Pittsburgh experience. Anesth Analg 2012; 115:678-88; PMID:22745115
- Gorantla VS, Brandacher G, Schneeberger S, Zheng XX, Donnenberg AD, Losee JE, Lee WP. Favoring the risk-benefit balance for upper extremity transplantation–the Pittsburgh Protocol. Hand Clin 2011; 27:511-20, ix-x; PMID:22051391; http://dx.doi.org/10.1016/j.hcl.2011.08.008
- Khalifian S, Raimondi G, Lee WA, Brandacher G. Taming inflammation by targeting cytokine signaling: new perspectives in the induction of transplantation tolerance. Immunotherapy 2014; 6:637-53; PMID:24896631; http://dx.doi.org/10.2217/imt.14.25
- Lin CH, Zhang W, Ng TW, Zhang D, Jiang J, Pulikkottil B, Lakkis F, Gorantla VS, Lee WP, Brandacher G, et al. Vascularized osteomyocutaneous allografts are permissive to tolerance by induction-based immunomodulatory therapy. Am J Transplant 2013; 13:2161-8; PMID:23718897; http://dx.doi.org/10.1111/ajt.12275
- Schneeberger S, Gorantla VS, Brandacher G, Zeevi A, Demetris AJ, Lunz JG, Metes DM, Donnenberg AD, Shores JT, Dimartini AF, et al. Upper-extremity transplantation using a cell-based protocol to minimize immunosuppression. Ann Surg 2013; 257:345-51; PMID:23001085; http://dx.doi.org/10.1097/SLA.0b013e31826d90bb
- Wachtman GS, Wimmers EG, Gorantla VS, Lin CH, Schneeberger S, Unadkat JV, Zheng XX, Brandacher G, Lee WP. Biologics and donor bone marrow cells for targeted immunomodulation in vascularized composite allotransplantation: a translational trial in swine. Transplant Proc 2011; 43:3541-4; PMID:22099837; http://dx.doi.org/10.1016/j.transproceed.2011.10.010
- Brandacher G, Lee WP, Schneeberger S. Minimizing immunosuppression in hand transplantation. Expert Rev Clin Immunol 2012; 8:673-83; quiz 84; PMID:23078064; http://dx.doi.org/10.1586/eci.12.54
- Leto Barone AA, Sun Z, Montgomery RA, Lee WP, Brandacher G. Impact of donor-specific antibodies in reconstructive transplantation. Expert Rev Clin Immunol 2013; 9:835-44; PMID:24070047; http://dx.doi.org/10.1586/1744666X.2013.824667
- Blume KR, Dietrich C, Huonker R, Gotz T, Sens E, Friedel R, Hofmann GO, Miltner WH, Weiss T. Cortical reorganization after macroreplantation at the upper extremity: a magnetoencephalographic study. Brain 2014; 137:757-69; PMID:24480484; http://dx.doi.org/10.1093/brain/awt366
- Kumnig M, Jowsey SG, Moreno E, Brandacher G, Azari K, Rumpold G, Group RTW. Case series on defense mechanisms in patients for reconstructive hand transplantation: consideration on transplant defense concept. Ann Transplant 2014; 19:233-40; PMID:24841554; http://dx.doi.org/10.12659/AOT.890326
- Carty MJ, Hivelin M, Dumontier C, Talbot SG, Benjoar MD, Pribaz JJ, Lantieri L, Pomahac B. Lessons learned from simultaneous face and bilateral hand allotransplantation. Plast Reconstr Surg 2013; 132:423-32; PMID:23584623; http://dx.doi.org/10.1097/PRS.0b013e318295883d
