Abstract
Stress response mediator activating transcription factor 3 (ATF3) engages in diverse oncogenic pathways including the androgen receptor signaling essential for prostatic proliferation. In line with frequent downregulation of ATF3 expression in human prostate cancers, we have provided the first genetic evidence supporting the role of ATF3 as a tumor suppressor in a subset of prostate cancers with PTEN dysfunction.
Keywords:
Activating transcription factor 3 (ATF3) is one of the smallest members of the ATF/CREB family of transcription factors, containing only one functional domain (the basic-region leucine zipper domain, bZIP) that binds the ATF/CREB cis-regulatory element (5’-TGACGTCA-3’) while dimerizing with other ATF/CREB proteins. The bZIP domain also mediates interactions with many other essential cellular proteins such as p53 (TP53, best known as p53), SMAD family member 3 (Smad3), and mouse double minute 2 (MDM2).Citation1-3 As ATF3 can be rapidly induced by a broad spectrum of cellular stresses (e.g., endoplasmic reticulum stress, oxidative stress, and DNA damage), it is generally believed that it plays important roles in the maintenance of genetic integrity and cellular homeostasis under stressed conditions. Indeed, ATF3 has been shown to engage in diverse cellular signaling pathways, including those mediated by transforming growth factor β (TGFβ), Toll-like receptor 4, and the tumor suppressor p53.Citation1,2,4 Not surprisingly, recent studies have unveiled crucial but controversial roles of ATF3 in human cancers.Citation5 For instance, although ATF3 was previously shown to be a metastasis promoter in a murine B16 melanoma model, we and others have demonstrated that ATF3 can suppress invasion and metastasis of lung and bladder cancers.Citation6,7 In a similar vein, it is reported that ATF3 induces apoptosis and restricts prostate cancer outgrowth while promoting invasion of prostate cancer cells.Citation5 Although it is highly possible that ATF3 plays a context-dependent role in cancer, these discrepancies might also reflect intrinsic differences in the cell models that were applied in different studies and that often fail to closely mimic physiological conditions. It is thus important to define the role of ATF3 in cancer using genetically engineered mouse models. In an early study, we examined a Atf3 knockout (Atf3−/−) mouse strain and found that Atf3 deficiency causes prostatic hyperplasia.Citation8 These results are consistent with observations that ATF3 binds the androgen receptor (AR) and represses the androgen signaling that is crucial for sustaining proliferation and survival of prostatic epithelial cells,Citation8 and suggest that it is more likely that ATF3 functions as a tumor suppressor for prostate cancer. Indeed, ATF3 expression is frequently downregulated in human prostate cancers.Citation8
To provide direct genetic evidence linking ATF3 to prostate cancer suppression, we crossed the Atf3−/− mice (ΔATF3) with phosphatase and tensin homolog (Pten) prostate-specific knockout mice (Ptenpc(−/−), ΔPten) to generate compound mutant mice (ΔATF3ΔPten).Citation9 Inactivation of PTEN by gene mutations or deletion leading to activation of oncogenic phosphoinositide-3-kinase/v-Akt murine thymoma viral oncogene homolog (PI3K/Akt) signaling is one of the most common genetic abnormalities in prostate cancers, occurring in approximately 30% of prostate tumors and up to 60% of metastatic prostate cancers. Interestingly, we found that the oncogenic stress triggered by loss of Pten in mouse prostatic epithelium induced expression of both ATF3 and p53, whereas p53 induction was completely abolished in ΔATF3 mice. These results are consistent with an early report that ATF3 can activate p53 in stressed conditions.Citation2 Although p53 activation did not appear to prevent prostate tumorigenesis in our mouse model, we found that the ΔATF3ΔPten mice developed prostatic intraepithelial neoplasia (PIN) and invasive adenocarcinoma at an earlier age and at a higher rate than the ΔPten mice. Staining for expression of α-smooth muscle actin—an invasion marker—confirmed that the number of invasive glands in the ΔATF3ΔPten mice was significantly increased compared with that in the ΔPten mice. Such effects appeared to be caused by increased proliferation and decreased apoptosis of prostatic epithelial cells. As Akt signaling can increase cell proliferation and promote cell survival, we examined Akt phosphorylation and activation in order to gain further insight into the underlying molecular mechanism by which Atf3 deficiency promotes prostate cancer development. Whereas loss of Pten indeed resulted in Akt activation, as evidenced by elevated Akt Ser473 and Thr308 phosphorylation levels, Atf3 deficiency dramatically increased Akt phosphorylation levels, indicating that ATF3 suppressed Akt activation in Pten-inactivated prostate lesions. To corroborate these important findings, we knocked down ATF3 expression in LNCaP, PC3, and DU145 human prostate cancer cells using the emerging CRISPR/Cas9 genome editing tool. We confirmed that ATF3 dysregulation could promote Akt signaling in prostate cancer cells. As Akt signaling is known to activate NF-κB and regulate expression of matrix metalloproteinases (MMPs) that drive cancer invasion and metastasis, we examined MMP expression and found that expression of MMP-2/MMP-9 was increased in the ATF3-downregulated human prostate cancer cells as well as in the Atf3-deficient mouse prostate lesions. Taken together, results presented in our recently published reportCitation9 demonstrated that loss of Atf3 promotes Akt activation and prostate cancer development. Therefore, we have provided the first genetic evidence supporting a role for ATF3 as a tumor suppressor for the major subset of prostate cancers harboring dysfunctional PTEN.
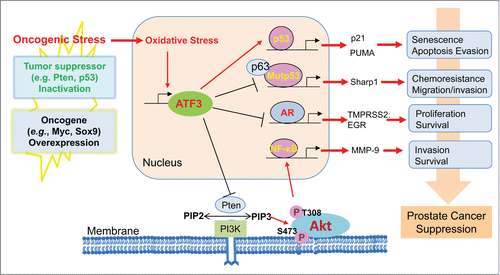
Oncogenic stress triggered by common genetic abnormalities in prostate cancers, such as oncogene overexpression (e.g., Myc and SRY-box 9 [Sox9]) or tumor suppressor inactivation (e.g., PTEN and p53) often generates oxidative stress, which can in turn induce ATF3 expression ().Citation5 Although we previously showed that ATF3 promotes p53 tumor suppressor activity,Citation2 we have also demonstrated that ATF3 counteracts chemoresistance and suppresses migration mediated by oncogenic p53 mutationsCitation6 thereby likely targeting metastatic prostate cancers that often carry p53 mutations. Although this notion remains to be demonstrated, our other studies showed that ATF3 is a physiologically relevant AR repressor that can repress the androgen signaling indispensable for the growth and survival of prostate cancer cells.Citation8 Although ATF3 regulates these cellular functions in the nucleus, our most recent studies have revealed a new role for ATF3 in regulating oncogenic signaling occurring in the cytoplasm (). As ATF3 is predominantly localized in the nucleus of prostate epithelial cells, how this nuclear protein suppresses Akt activation occurring in the cytoplasm remains elusive. It is possible that, as a transcriptional factor, ATF3 alters the expression of one or a set of major Akt regulators such as PH domain and leucine-rich repeat protein phosphatases (PHLPPs) and phosphoinositide-dependent kinase-1 (PDK1). Alternatively, ATF3 might also directly interact with these regulators to modulate their functions. Regardless of the mechanism, results of this recent study combined with our earlier findings suggest that ATF3 induced by oncogenic stress can contribute to suppression of prostate cancer (). It is important to note that recent studies highlight the importance of combined inhibition of PI3K/Akt and AR signaling in the treatment of patients with prostate cancer due to reciprocal feedback regulation of these 2 oncogenic signaling pathways.Citation10 As ATF3 can suppress both PI3K/Akt and AR signaling, targeting ATF3 might serve as a promising strategy for therapeutic intervention of prostate cancer.
Figure 1. Contribution of activating transcription factor 3 (ATF3) to the suppression of prostate cancer. In addition to regulating the pathways mediated by wild-type and mutant p53 proteins, ATF3 can also suppress androgen receptor signaling while promoting Akt activation in response to the oncogenic stress triggered by inactivation of tumor suppressors and/or overexpression of oncogenes.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by NIH/NCI grants R01CA139107 and R01CA164006 to CY.
References
- Kang Y, Chen C, Massague J. A self-enabling TGFß response coupled to stress signaling: smad engages stress response factor ATF3 for Id1 repression in epithelial cells. Mol Cell 2003; 11:915-26; PMID:12718878; http://dx.doi.org/10.1016/S1097-2765(03)00109-6
- Yan C, Lu D, Hai T, Boyd DD. Activating transcription factor 3, a stress sensor, activates p53 by blocking its ubiquitination. EMBO J 2005; 24:2425-35; PMID:15933712; http://dx.doi.org/10.1038/sj.emboj.7600712
- Mo P, Wang H, Lu H, Boyd DD, Yan C. MDM2 mediates ubiquitination and degradation of activating transcription factor 3. J Biol Chem 2010; 285:26908-15; PMID:20592017; http://dx.doi.org/10.1074/jbc.M110.132597
- Gilchrist M, Thorsson V, Li B, Rust AG, Korb M, Roach JC, Kennedy K, Hai T, Bolouri H, Aderem A. Systems biology approaches identify ATF3 as a negative regulator of toll-like receptor 4. Nature 2006; 441:173-8; PMID:16688168; http://dx.doi.org/10.1038/nature04768
- Yan C, Boyd DD. ATF3 regulates the stability of p53: a link to cancer. Cell Cycle 2006; 5:926-929; PMID:16628010; http://dx.doi.org/10.4161/cc.5.9.2714
- Wei S, Wang H, Lu C, Malmut S, Zhang J, Ren S, Yu G, Wang W, Tang DD, Yan C. The activating transcription factor 3 protein suppresses the oncogenic function of mutant p53 proteins. J Biol Chem 2014; 289:8947-59; PMID:24554706; http://dx.doi.org/10.1074/jbc.M113.503755
- Yuan X, Yu L, Li J, Xie G, Rong T, Zhang L, Chen J, Meng Q, Irving AT, Wang D., et al. ATF3 suppresses metastasis of bladder cancer by regulating gelsolin-mediated remodeling of the actin cytoskeleton. Cancer Res 2013; 73:3625-37; PMID:23536558; http://dx.doi.org/10.1158/0008-5472.CAN-12-3879
- Wang H, Jiang M, Cui H, Chen M, Buttyan R, Hayward SW, Hai T, Wang Z, Yan C. The stress response mediator ATF3 represses androgen signaling by binding the androgen receptor. Mol Cell Biol 2012; 32 3190-202; PMID:22665497; http://dx.doi.org/10.1128/MCB.00159-12
- Wang Z, Xu D, Ding H.-F, Kim J, Zhang J, Hai T, Yan C. Loss of ATF3 promotes akt activation and prostate cancer development in a pten knockout mouse model. Oncogene 2014; PMID:25531328; http://dx.doi.org/10.1038/onc.2014.426
- Carverm BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H., et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 2011; 19 575-86; PMID:21575859; http://dx.doi.org/10.1016/j.ccr.2011.04.008
