ABSTRACT
Ultraviolet radiation (UVR) instantaneously generates cyclobutane pyrimidine dimers (CPDs). Paradoxically, we recently observed that UV enables the protective pigment melanin to create CPDs in the dark long after the exposure ends. UV-induced reactive oxygen species (ROS) oxidize melanin to create melanin carbonyls in a high-energy quantum state. These energetic melanin carbonyls transfer their energy to DNA in the dark, creating CPDs in the absence of UVR.
Mutations present in sunlight-induced melanoma and other skin cancers are often cytosine (C) to thymine (T) or CC to TT base substitutions at DNA sites where 2 pyrimidines (cytosine or thymine) are next to each other.Citation1 These mutations arise at the sites of cyclobutane pyrimidine dimers (CPDs), photoproducts that are normally created almost instantaneously when DNA absorbs an ultraviolet (UV) photon. These CPDs are slowly repaired by nucleotide excision repair. Our recent study discovered that, while this is true for fibroblasts and albino melanocytes, melanin-containing melanocytes continue to generate CPDs for several hours in the dark (termed “dark CPDs”) in response to UVA (∼320–400 nm) or UVB (˜280–320 nm) exposure.Citation2 This phenomenon was observed in vivo in UVA-exposed mouse skin, as well as in mouse and human melanocytes in cell culture. DNA has poor absorption for UVA, which creates primarily TT CPDs. We observed a considerably higher ratio of TC+CT/TT substitutions for dark CPDs, which was both intriguing and concerning because cytosine-containing CPDs are frequently associated with melanoma.
Melanin acts as a potent sun shield,Citation3 thus the fact that dark CPDs were generated only in melanin-containing cells was unforeseen and directly implicated the involvement of melanin. UVR upregulates melanin synthesisCitation4 () and this synthesis pathway, especially that for pheomelanin, generates reactive oxygen species (ROS).Citation5 Inhibition of melanin synthesis significantly reduces the generation of dark CPDs. Additionally, UV radiation (UVR) activates inducible nitric oxide synthase (iNOS) and NADPH oxidase (NOX), which generate nitric oxide and superoxide, respectivelyCitation6,7 (). Treatment with iNOS and NOX inhibitors and ROS scavengers inhibits the generation of dark CPDs.Citation2 Combined, these findings demonstrated a combined role of melanin and ROS in the generation of dark CPDs.
Figure 1. Putative role of melanin in the generation of dark CPDs. Ultraviolet (UV) exposure induces melanin synthesis, during which melanin monomers accumulate along the nuclear membrane. Direct UV exposure of melanin in melanosomes (“M”, black circles) breaks down the melanin into monomers (small black circles). The melanin monomers are lipophilic and can cross the nuclear membrane (broken black arrows). Melanin synthesis also produces superoxide (O2·−) which, owing to its close proximity to the nuclear membrane, can presumably cross into the nucleus (purple broken arrows). UV exposure also induces the production of superoxide anions and nitric oxide (NO·) radicals by respectively activating NADPH oxidase and iNOS enzymes. Superoxide and nitric oxide combine with each other to make peroxynitrite (ONOO−). Both nitric oxide and peroxynitrite can diffuse intracellularly or across the cell membrane. Superoxide and nitric oxide can also generate peroxynitrite in the nucleus, where it reacts with melanin monomers to produce melanin carbonyls (C=O, highlighted in green) in excited triplet states. An excited triplet carbonyl transfers its energy to a DNA dipyrimidine (Py), creating a cyclobutane pyrimidine dimer (CPD).
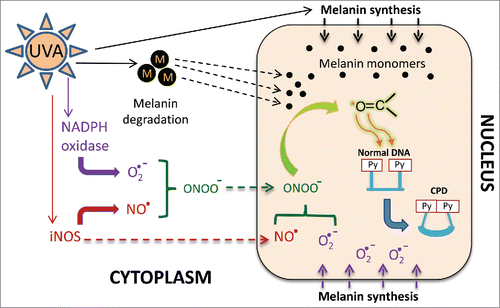
The involvement of superoxide and nitric oxide was fascinating since their combination produces peroxynitrite, which can form 4-membered strained ring structures with 2 oxygen and 2 carbon atoms. These structures, called dioxetanes, have been shown to promote the formation of CPDs in the dark.Citation8 A dioxetane undergoes thermolysis to create 2 carbonyls. One of these carbonyls is in an excited triplet state that possesses more energy than its ground state. This energy can then be absorbed by DNA, generating CPDs.Citation8 Dioxetanes can be generated through cycloaddition at enols and indoles, which are abundant in the melanin synthesis pathway. ROS that can potentially generate dioxetanes include peroxynitrite and lipid peroxides. Although there was no detectable lipid peroxidation, we did observe endogenous nuclear peroxynitrite, specifically in melanin-containing melanocytes. Moreover, there was an additional ∼400-fold burst in nuclear peroxynitrite levels after UVA exposure. This observation made us wonder whether melanin and its monomers react with the UVA-induced peroxynitrite to generate dioxetanes, and thus triplet carbonyls, in melanin-containing melanocytes. Ultraweak chemiluminescence, which is the emission of low levels of light from a chemical reaction, is one of the molecular diagnostic tools for excited triplet carbonyls. Surprisingly, melanin-containing melanocytes generate luminescence in response to UVA.Citation2 Furthermore, indisputable evidence of excited triplet carbonyls came from inhibition of dark CPDs in the presence of sorbate, a specific quencher of triplet energy.Citation9 Addition of 9,10-dibromoanthracene-2-sufonate (DBAS), which diverts the energy of excited triplets to luminescence,Citation9 also reduced the number of dark CPDs. Moreover, cell-free oxidation of synthetic melanin with peroxynitrite, or with hydrogen peroxide and peroxidase, generated ultraweak chemiluminescence. Finally, the cell-free melanin oxidation reaction also generated CPDs in co-incubated plasmid DNA.
These experiments demonstrated that excited triplet carbonyls generated from melanin oxidation are responsible for the generation of dark CPDs (). However, since melanin is located in the cytoplasm the molecular vector carrying the triplet carbonyls across the nuclear membrane was still unknown. A ROS-initiated radical chain reaction through membrane lipids was one possibility, but lipid peroxidation using cumene hydroperoxide did not yield any dark CPDs.Citation2 Another possible vector was melanin monomers, which are lipophilic and might enter the nucleus. Melanin synthesis occurs along the nuclear membrane, where the monomers are oxidized by tyrosinase to initiate their polymerization. The close proximity of melanin synthesis and the nuclear membrane further increases the probability of lipophilic melanin monomers entering into the nucleus (). We also observed that UVA is able to solubilize melanin polymerCitation2 and the resulting melanin derivatives and monomers can diffuse intracellularly, potentially entering the nucleus. In addition, peroxynitrite and nitric oxide can diffuse across membranes and there are reports of NOX4 in the nucleus,Citation7 which would generate superoxide. This series of events would lead to the generation of dioxetanes on the melanin derivatives in the nucleus. These dioxetanes undergo thermolysis and the resulting excited triplet carbonyl transfers the energy to pyrimidines in the DNA to generate dark CPDs (). The fact that the activity of NOX and iNOS persisted long after UVR exposure ended suggests ongoing creation of dioxetanes and their thermolysis, visible in the form of ultraweak chemiluminescence from the resulting triplet carbonyl. To identify the melanin derivative carrying the triplet carbonyl moiety, we oxidized a synthetic monomer of melanin, 5, 6-dihydroxyindole-2-carboxylic acid (DHICA) with hydrogen peroxide and peroxidase. Mass spectrometric analysis of DHICA oxidation products suggested that dioxetane generation and resulting carbonyl occur on the 5-membered pyrrole ring of DHICA.Citation2
As melanin has been perceived as a sun shield, its role in inducing carcinogenic CPDs through excited melanin carbonyls was an unpleasant surprise. Research on excited triplet carbonyls is evidently important, yet rarely undertaken. Our discovery of melanin carbonyls directly validated the importance of chemically excited species in mammalian biology. Reports on the apoptogenic role of carbonyl scavengers, specifically in melanoma,Citation10 further emphasize the need for a better understanding of excited and non-excited carbonyls. Fortunately, the dark photochemistry is slow and allows intervention, as we demonstrated using ROS scavengers, enzyme inhibitors, and triplet energy quenchers. We suggest screening for efficient triplet energy quenchers that might be formulated to make “evening-after sunscreens.”
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The authors' research was supported by Department of Defense CDMRP grant CA093473P1 and NIH grant 2 P50 CA121974.
References
- Brash DE. UV signature mutations. Photochem Photobiol 2015; 91:15-26; PMID:25354245; http://dx.doi.org/10.1111/php.12377.
- Premi S, Wallisch S, Mano CM, Weiner AB, Bacchiocchi A, Wakamatsu K, Bechara EJ, Halaban R, Douki T, Brash DE. Chemiexcitation of melanin derivatives induce DNA photoproducts long after UV exposure. Science 2015; 347(6224):842-7; PMID:25700512; http://dx.doi.org/10.1126/science.1256022.
- Kollias N, Sayre RM, Zeise L, Chedekel MR. Photoprotection by melanin. J Photochem Photobiol 1991; 9(2):135-60; PMID:1907647; http://dx.doi.org/10.1016/1011-1344(91)80147-A.
- Carsberg CJ, Warenius HM, Friedmann PS. Ultraviolet radiation-induced melanogenesis in human melanocytes. Effects of modulating protein kinase C. J cell Sci 1994; 107(Pt9):2591-7; PMID:7531203.
- Munoz-Munoz JL, Garcia-Molina F, Varon R, Tudela J, Garcia-Canovas F, Rodriguez-Lopez JN. Generation of hydrogen peroxide in melanin biosynthesis pathway. Biochim Biophys Acta 2009; 1794(7):1017-29; PMID:19374959; http://dx.doi.org/10.1016/j.bbapap.2009.04.002.
- Valencia A, Kochevar IE. Nox-1 based NADPH oxidase is the major source of UVA-induced reactive oxygen species in human keratinocytes. J Invest Dermatol 2008; 128:214-222; PMID:17611574; http://dx.doi.org/10.1038/sj.jid.5700960.
- Romero-Graillet C, Aberdam E, Biagoli N, Massabni W, Ortonne JP, Ballotti R. Ultraviolet B radiation acts through the nitric oxide and Cgmp signal transduction pathway to stimulated melanogenesis in human melanocytes. J Biol Chem 1996; 271(45):28052-6; PMID:8910416; http://dx.doi.org/10.1074/jbc.271.45.28052.
- Lamola AA. Production of pyrimidine dimers in the dark. Biochem Biophys Res Commun 1971; 43:893-8; PMID:4935292; http://dx.doi.org/10.1016/0006-291X(71)90701-7.
- Velosa AC, Baader WJ, Stevani CV, Mano CM, Bechara EJ. One-3-diene probes for detection of triplet carbonyls in biological systems. Chem Res Toxicol 2007; 20:1162-9; PMID:17630714; http://dx.doi.org/10.1021/tx700074n.
- Wondrak GT, Jacobson MK, Jacobson EL. Antimelanoma activity of apoptogenic carbonyl scavengers. J Pharmacol Exp Ther 2006; 316(2):805-14; PMID:16210394; http://dx.doi.org/10.1124/jpet.105.094953.
