ABSTRACT
We have identified a novel mechanism of regulation of the protein CDKN1A (also known as p21) by the serine/threonine kinase complex mammalian target of rapamycin complex 1 (mTORC1). Our results demonstrate that the mTORC1 substrate EIF4E-binding protein 1 (4E-BP1) in its non-phosphorylated state interacts with p21 and promotes p21 degradation. In addition, we demonstrate the prevalence of this mechanism in head and neck squamous cell carcinomas and show that it strongly and significantly associates with improved disease-specific survival, providing evidence for its clinical relevance.
The protein p21, encoded by the CDKN1A gene, is a member of the CIP/KIP family of cyclin-dependent kinase (CDK) inhibitors that govern cell cycle progression in eukaryotic cells. The expression of p21 is mainly regulated at the transcriptional level by the tumor-suppressor protein TP53 (best known as p53); however, additional mechanisms contributing to the regulation of p21 cellular levels have been described.Citation1,2
The serine/threonine kinase complex mammalian target of rapamycin complex 1 (mTORC1) promotes anabolic processes and cell growth in response to environmental cues. When activated, mTORC1 increases mRNA translation and protein synthesis through the phosphorylation of at least 2 substrates: the kinase S6 (S6K) and the translational regulator EIF4E-binding protein 1 (4E-BP1). Upon phosphorylation, 4E-BP1 releases the translational helicase eIF4A, allowing it to initiate translation of a subset of mRNAs known as TOP mRNAs that are characterized by the presence of a terminal oligopyrimidine (TOP) track in the 5′-untranslated region.Citation3,4
Previous studies have linked p21 levels and mTORC1 activity through different mechanisms such as upregulation of the translation of p21 and p53 mRNAsCitation5,6 or activation of p53 via inhibition of its E3 ubiquitin ligase mouse double minute 2 (MDM2).Citation7 In addition, the global effect of prolonged mTORC1 inhibition on mRNA translation has been proposed to have a greater impact on short-lived proteins such as p21.Citation8
We found that mTORC1 hyperactivation by cellular depletion of its inhibitor tuberous sclerosis 2 (TSC2) caused a robust upregulation of p21 levels in both wild type and p53-null cells, implicating a p53-independent mechanism. TSC2 silencing also increased the expression of ectopic p21 under the control of a heterologous promoter, suggesting the involvement of post-transcriptional regulation. Furthermore, ectopic p21 contained an open reading frame preceded by a heterologous 5′UTR sequence, indicating that control of p21 mRNA translation was not responsible for the effect of mTORC1 on p21 expression. This was supported by the observation that eIF4E-specific shRNAs, which prevent TOP mRNA translation, failed to antagonize mTORC1-mediated upregulation of p21. Finally, we found that TSC2 depletion significantly extended the p21 protein half-life. These observations collectively indicate that mTORC1 regulates p21 stability. The finding that a 4E-BP1 phosphorylation-defective mutant prevented p21 upregulation upon TSC2 depletion pointed to a role as an important mediator of the mTORC1 effects on p21. Our study further revealed that p21 interacts with 4E-BP1 only in the presence of the mTOR inhibitor Torin-1. Taken together, these results are consistent with a model in which under basal conditions non-phosphorylated 4E-BP1 associates with p21 and promotes its degradation. Conversely, upon mTORC1 activation, phosphorylation of 4E-BP1 abrogates its binding to p21 leading to accumulation of p21 protein.
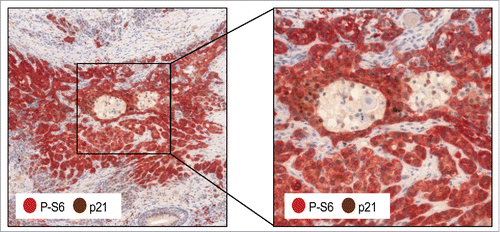
We have found that the mTORC1/4EBP1/p21 regulatory axis is highly prevalent in head and neck squamous cell carcinomas (HNSCC), in which p53 mutations are frequent and widespread genomic and epigenetic alterations of several components of the mTORC1 pathway are consistently observed.Citation9 HNSCC encompasses a group of heterogeneous cancers arising mainly in the oral cavity, oropharynx, larynx, or hypopharynx. This malignancy constitutes the sixth most prevalent cancer worldwide and despite advances in treatments the 5-year survival rate for patients with HNSCC remains disappointingly low. p21 expression is frequently detected in HNSCC irrespective of p53 status. By analyzing a large series of 274 tumor samples from HNSCC patients, we found that p21 expression strongly and significantly correlated with S6 phosphorylation (P-S6) (), which we used as readout for mTORC1 activity. This observation strongly suggests that p21 overexpression is secondary to mTORC1 activation in these tumors. Co-immunodetection of p21 and P-S6 was found in a considerable percentage of tumors (62%) and, more importantly, was linked to significantly improved disease-free survival. Moreover, the subgroup of patients with p21/P-S6 double-positive tumors was also frequently and significantly associated with the absence of lymph node metastasis at the time of diagnosis, and consequently with a less aggressive disease. These findings unveil the clinical and pathobiological relevance of the mTORC1/4E-BP1/p21 regulatory axis in HNSCC and its impact on the prognosis of these patients.
Figure 1. Co-expression of p21 and phosphorylated S6 in head and neck squamous cell carcinoma (HNSCC). Representative section from a HNSCC tissue sample showing immunohistochemical detection of CDKN1A/p21 and phosphorylated-S6 (P-S6).
At present, the underlying reasons for the slower progression of p21/P-S6 positive tumors are unclear. In agreement with previous reports, we detected p21 expression in proliferating HNSCC cells, indicating that the growth-inhibitory function of p21 is abrogated in these tumors and does not account for its protective role. Nevertheless, p21 has been shown to be involved in additional cellular processes such as differentiation, apoptosis, and stem cell quiescence,Citation1,2 which could also have an impact on tumor progression.
It is important to note that the association of mTORC1 activation in HNSCC with better prognosis is not in conflict with the oncogenic role of mTORC1 in these cancers, or with the potential therapeutic value of mTOR inhibitors for HNSCC.
These results, recently published in Nature Communications (Llanos et al., 2016), contribute to improved understanding of p21 regulation in cancer cells and provide new tools that could help clinicians to establish prognosis more accurately in HNSCC patients and guide treatment decisions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Warfel NA, El-Deiry WS. p21WAF1 and tumourigenesis: 20 years after. Curr Opin Oncol 2013; 25:52-8; PMID:23159848; http://dx.doi.org/10.1097/CCO.0b013e32835b639e.
- Jung YS, Qian Y, Chen X. Examination of the expanding pathways for the regulation of p21 expression and activity. Cell Signal 2010; 22:1003-12; PMID:20100570; http://dx.doi.org/10.1016/j.cellsig.2010.01.013.
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol 2009; 10:307-18; PMID:19339977; http://dx.doi.org/10.1038/nrm2672
- Liu Q, Chang JW, Wang J, Kang SA, Thoreen CC, Markhard A, Hur W, Zhang J, Sim T, Sabatini DM, et al. Discovery of 1-(4-(4-propionylpiperazin-1-yl)-3-(trifluoromethyl)phenyl)-9-(quinolin-3-yl)benz o[h][1,6]naphthyridin-2(1H)-one as a highly potent, selective mammalian target of rapamycin (mTOR) inhibitor for the treatment of cancer. J Med Chem 2010; 53:7146-55; PMID:20860370; http://dx.doi.org/10.1021/jm101144f.
- Tahmasebi S, Alain T, Rajasekhar VK, Zhang J, Prager-khoutorsky M, Khoutorsky A, Dogan Y, Gkogkas CG, Petroulakis E, Sylvestre A, et al. Article Multifaceted regulation of somatic cell reprogramming by mrna translational control. Cell Stem Cell 2013; 14(5):606-16; PMID:24630793; http://dx.doi.org/10.1016/j.stem.2014.02.005
- Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest 2010; 120:681-93; PMID:20197621; http://dx.doi.org/10.1172/JCI40535.
- Lai KP, Leong WF, Chau JFL, Jia D, Zeng L, Liu H, He L, Hao A, Zhang H, Meek D, et al. S6K1 is a multifaceted regulator of Mdm2 that connects nutrient status and DNA damage response. EMBO J 2010; 29:2994-3006; PMID:20657550; http://dx.doi.org/10.1038/emboj.2010.166.
- Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O’Reilly T, Natt F, Hall J, Lane HA, Thomas G. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell 2005; 120:747-59; PMID:15797377; http://dx.doi.org/10.1038/nrc2982
- Leemans CR, Braakhuis BJM, Brakenhoff RH. The molecular biology of head and neck cancer. Nat Rev Cancer 2011; 11:9-22; PMID:21160525; http://dx.doi.org/10.1038/nrc2982.
