ABSTRACT
Besides being a critical tumor suppressor, the TP53 gene also plays a role in metabolism and recent studies in humans have implicated the codon 72 polymorphism of TP53 in this role. Using a humanized knock-in mouse model for these TP53 variants, we show that this polymorphism has a significant impact on the metabolic response to a high-fat diet.
Two puzzling scientific mysteries, one in human evolution and the other in cancer biology, have converged at an unexpected intersection. Since its discovery nearly 40 years ago, TP53 (more commonly known as p53) has been established as a critical tumor suppressor in human cancer. Functioning primarily as a transcription factor, p53 inhibits tumor development by regulating the expression of genes associated with programmed cell death (apoptosis), cell cycle arrest, and senescence. Dysregulation of the p53 pathway occurs in the majority of all cancers, among which more than 50% possess somatic point mutations of p53.Citation1
In addition to somatic mutations that occur in cancer, the TP53 gene also contains single nucleotide polymorphisms, or SNPs, that alter the coding region of the protein. The most common SNP occurs at codon 72 of p53 and changes the ancestral proline residue (P72) to an arginine (R72). Interestingly, this SNP displays distinct distribution patterns among racial and ethnic groups that may be geographically influenced. In the United States, the R72 variant is markedly more prevalent in Caucasian Americans compared to African Americans. Furthermore, the frequency of R72 increases in a linear manner with latitude. These correlations have prompted speculation that this polymorphism arose due to natural selection in response to stresses such as UV exposure or colder winter temperatures.Citation2 However, underlying differences in function between these variants, and the possible reason(s) why the R72 variant arose, have remained a mystery.
Like cancer, diabetes and obesity are worldwide epidemics with known genetic components. And, like p53 polymorphic variants, the possible selection pressure that might have led to the increased incidence of diabetes and obesity has been the target of considerable speculation, among which is the controversial “thrifty gene” hypothesis. First proposed by James Neel in 1962, this hypothesis states that genetic variants that predispose to diabetes and obesity may have been selected for in early humans in order to help them deposit fat more efficiently, and thus survive better in times of famine. These genetic variants, which at one time were historically advantageous, later became detrimental in the modern world.Citation3
These two seemingly unrelated phenomena became linked in 2008, when an unbiased screen revealed that the R72 variant of p53 is one of the strongest genetic variants associated with diabetes.Citation5 This association was later confirmed in a meta-analysis consisting of over 55,000 northern Europeans.Citation6 To better understand the molecular basis for this association, we utilized a mouse model in which exons 4–9 of murine p53 were replaced by human p53 exons containing either the P72 or R72 variant and investigated the impact of this polymorphism on the response to a normal or high-fat diet (HFD).Citation7 Under the normal diet, R72 mice gained slightly more weight than P72 mice, but otherwise had similar metabolic profiles. In contrast, following a HFD, R72 mice gained up to 20% more weight and developed a variety of prediabetic metabolic symptoms, including insulin resistance, pancreatic hypertrophy, and non-alcoholic fatty liver disease. By performing gene expression profiling of p53-regulated genes in the livers of mice following a HFD, we observed that R72 livers showed increased induction of some proinflammatory p53-regulated genes, as well as dysregulation of p53-regulated genes involved in lipid metabolism.
To narrow in on the candidate genes responsible for the increased weight gain and prediabetic syndromes in R72 mice, we repeated the gene expression analysis in livers of mice following a short-term HFD for 7 days. In this analysis 2 genes were significantly upregulated in R72 livers compared to P72 livers: Tnf (tumor necrosis factor) and Npc1l1 (NPC1-like 1). Npc1l1 is a known p53 target gene that plays a role in cholesterol metabolism. Although there have been many reports speculating that Tnf is a p53 target gene, binding of p53 to a consensus binding site in the upstream regulatory region of this gene has never been demonstrated. We showed that the R72 protein bound to p53 binding sites in both genes, and furthermore that it showed increased binding compared to the P72 variant. Moreover, by inhibiting the function of Tnf and Npc1l1 using antibodies or small-molecule inhibitors we were able to prevent the increased fat accumulation in livers of R72 mice following a HFD.
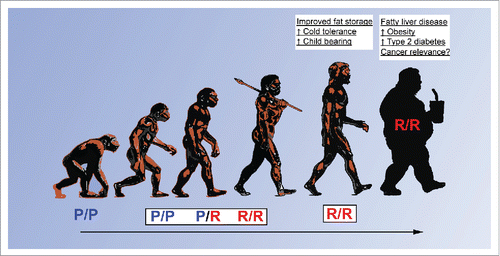
Our study not only uncovered a potential mechanism underlying the association between the codon 72 polymorphism of p53 and diabetes, but also offers a possible explanation for the selection pressure for this SNP in northern latitudes. The ancestral P72 variant of p53 is the only form found in primates, and is the most common variant in humans near the equator (). We posit that the R72 variant arose and was selected for in humans as they migrated north in order to provide more efficient fat accumulation, which would favor survival and fertility in colder temperatures. Interestingly, associations between R72 and colder winter temperatures and increased fertility have been noted previously.Citation8,9 In the modern era, without the threat of famine in colder temperatures, individuals with the R72 variant would be predicted to be more susceptible to increased weight gain, thereby explaining recent findings of an association of R72 with diabetes. From a medical standpoint, understanding the basis for this role of p53 in metabolism could help diagnose and treat R72 individuals. Moreover, given the known role of obesity in predisposing to cancer, these findings may also help explain the weak but persistent association of this codon 72 SNP with the risk for some, but not all, cancers. In light of these findings it will be interesting to determine whether this SNP plays a role in cancers known to be linked to metabolic dysfunction, such as endometrial cancer or post-menopausal breast cancer.Citation10 These hypotheses await testing.
Figure 1. Potential role for TP53 polymorphisms in human evolution. Proline at amino acid 72 (P72) of p53 is the ancestral variant and this form is found exclusively in primates. During the emergence of prehistoric humans, the arginine 72 form (R72) evolved from P72 and may have provided increased fat accumulation, which not only improved survival but also offered benefits such as enhanced cold tolerance and fertility. These benefits are diminished in the modern era due to industrialization and abundance of food sources, resulting in increased susceptibility to metabolic diseases such as obesity and type 2 diabetes.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by National Institutes of Health grant CA102184 (to M.M.) and Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute.
References
- Levine AJ, Oren M. The first 30 years of p53: growing ever more complex. Nat Rev Cancer 2009; 9:749-758; PMID:19776744; http://dx.doi.org/10.1038/nrc2723
- Murphy ME. Polymorphic variants in the p53 pathway. Cell Death Differentiation 2006; 13:916-920; PMID:16557270; http://dx.doi.org/10.1038/sj.cdd.4401907
- NEEL JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962 Dec;14:353-62. PubMed PMID: 13937884
- Bonnefond A, Froguel P. Rare and Common Genetic Events in Type 2 Diabetes: What Should Biologists Know? Cell Metab 2015; 21(3):357-68; PMID:25640731; http://dx.doi.org/10.1016/j.cmet.2014.12.020
- Gaulton KJ, Willer CJ, Li Y, Scott LJ, Conneely KN, Jackson AU, Duren WL, Chines PS, Narisu N, Bonnycastle LL, et al. Comprehensive association study of type 2 diabetes and related quantitative traits with 222 candidate genes. Diabetes 2008; 57:3136-3144; PMID:18678618; http://dx.doi.org/10.2337/db07-1731
- Burgdorf KS, Grarup N, Justesen JM, Harder MN, Witte DR, Jørgensen T, Sandbæk A, Lauritzen T, Madsbad S, Hansen T, et al. Studies of the association of Arg72Pro of tumor suppressor protein p53 with type 2 diabetes in a combined analysis of 55,521 Europeans. PloS One 2011; 6:e15813; PMID:21283750; http://dx.doi.org/10.1371/journal.pone.0015813
- Kung CP, Leu JI, Basu S, Khaku S, Anokye-Danso F, Liu Q, George DL, Ahima RS, Murphy ME. The P72R Polymorphism of p53 Predisposes to Obesity and Metabolic Dysfunction. Cell Rep 2016; 14:2413-2425; PMID:26947067; http://dx.doi.org/10.1016/j.celrep.2016.02.037
- Shi H, Tan SJ, Zhong H, Hu W, Levine A, Xiao CJ, Peng Y, Qi XB, Shou WH, Ma RL, et al. Winter temperature and UV are tightly linked to genetic changes in the p53 tumor suppressor pathway in Eastern Asia. Am J Hum Gen 2009; 84:534-541; PMID:19344876; http://dx.doi.org/10.1016/j.ajhg.2009.03.009
- Levine AJ, Tomasini R, McKeon FD, Mak TW, Melino G. The p53 family: guardians of maternal reproduction. Nature reviews. Mol Cell Biol 2011; 12:259-265; PMID:21427767; http://dx.doi.org/10.1038/nrm3086.
- Park J, Morley TS, Kim M, Clegg DJ, Scherer PE. Obesity and cancer–mechanisms underlying tumour progression and recurrence. Nature reviews. Endocrinology 2014; 10:455-465; PMID:24935119; http://dx.doi.org/10.1038/nrendo.2014.94
