ABSTRACT
Human pluripotent stem cells (hPSCs) frequently acquire chromosomal aberrations, including aneuploidy, during culture. Recently, we identified a replication stress-based mechanism leading to ongoing chromosomal instability in aneuploid hPSCs that may also operate during the initiation of instability in diploid cells.
Chromosomal instability is a hallmark of nearly all solid tumors and adult-onset leukemias. In recent years it has become apparent that this DNA instability is caused by DNA replication stress during the early stages of cancer development.Citation1,2 Replication stress is broadly defined as perturbations in the dynamics of the replication machinery, characterized by slow progression of DNA replication forks, replication fork arrest and even collapse, and activation of additional origins of DNA replication as an attempt of the cells to compensate for the slow rate. The presence of incompletely duplicated loci in metaphase results in chromosome mis-segregation and non-disjunction and therefore drives both structural and numerical chromosomal instabilities. Replication is frequently challenged through exogenously induced (e.g., low nutrient environment)Citation3 or endogenously induced (e.g., oncogene expression)Citation1 replication stress. Replication stress normally activates the DNA damage response (DDR) to induce cell cycle arrest. However, in the absence of functional cell cycle checkpoints, as in cancer cells, the DDR does not activate cell cycle arrest and the cells continue to proliferate despite unreplicated loci and DDR activation. Indeed, understanding the pathways that allow cancer cells to tolerate ongoing replication stress is highly important since targeting these pathways is emerging as a promising powerful therapeutic approach to fight cancer.
In addition to cancer cells, another group of cells that is characterized by low efficiency of cell cycle checkpoints activation is human embryonic pluripotent stem cells (hPSCs). Both mouse and human PSCs are characterized by an inefficient G1/S checkpoint arrest upon ionizing radiation or replication stress (reviewed in ref. 4Citation4) that is required to prevent cells with damaged DNA from entering the S phase. In addition, the G2/M decatenation checkpoint, which delays entry into mitosis until the chromosomes have been decatenated and untangled, is inefficient in hPSCs. PSCs are also characterized by a substantially shorter cell cycle, mainly due to a truncated G1 phase compared to committed and differentiated cells (reviewed in ref. 4Citation4). The unique cell cycle and checkpoint activation features of hPSCs may render them more susceptible than other cell types to genomic abnormalities from single nucleotide point mutations to full chromosome aneuploidy (numerical chromosomal aberrations). Interestingly, numerical aberrations in hPSCs tend to result in the acquisition of defined chromosomal aneuploidies. Typical changes are additional copies of chromosomes 1, 12, 17, and X, similar to changes found in germ cell tumors such as malignant embryonic carcinoma.Citation5 Indeed, hPSCs harboring these recurrent aneuploidies show higher tumorigenic potential and ongoing chromosomal instability similar to chromosome instability (CIN+) cancer cells. Therefore, we were intrigued to investigate the cellular mechanisms underlying the ongoing instability and tumorigenicity induced by these aneuploidies.Citation6
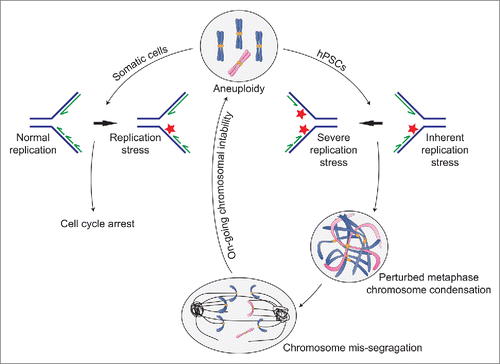
Aneuploid hPSCs are highly sensitive to replication inhibitors.Citation7 Therefore, we set out to study replication dynamics in aneuploid hPSCs by taking advantage of the DNA combing method. We found that aneuploid hPSCs (harboring trisomy 12, 17, or both) exhibit inherent perturbations of DNA replication characterized by a slow replication rate and activation of dormant origins.Citation6 Interestingly, a recent study showed that aneuploidy also induces replication stress in somatic cells.Citation8 However, unlike somatic cells, replication stress in hPCSs caused either by inherent stress (aneuploidy) or induced stress (aphidicolin) results in metaphase condensation failure and entangled chromosomes.Citation6 Chromosome separation in the presence of entangled chromosomes naturally leads to uneven segregation that forms the basis of ongoing instability in aneuploidy hPSCs. Notably, perturbations in metaphase chromosome condensation under normal culture conditions were highly prevalent in aneuploid hPSCs (37%), but were also common in diploid hPSCs (20%) compared to somatic cells (less than 5%). This implies that diploid hPSCs experience inherent replication stress that is further enhanced in aneuploid cells (). Indeed, 2 recent studies suggested that both mouse PSCs and induced-PSCs (iPSCs) experience constitutive replication stress,Citation9,10 suggesting that a process similar to the replication-based mechanism that leads to ongoing instability in aneuploid cells also drives instability in diploid cells ().
Figure 1. Effects of aneuploidy-induced replication stress on genome stability. A summary of recent findings showing the effects of aneuploidy and replication stress on cell cycle progression and genome stability in somatic cells and human pluripotent stem cells (hPSC). Aneuploidy leads to replication stress in somatic cells that is exacerbated by the inherent replication stress in hPSCs. However, whereas in normal somatic cells replication stress leads to cell cycle arrest, in hPSCs it fails to activate cell cycle checkpoints. Furthermore, due to an inefficient decatenation checkpoint, hPSCs proceed with mitosis in the presence of entangled chromosomes. This leads to anaphase abnormalities including anaphase bridges and lagging chromosomes that result in segregation errors and further chromosomal instability.
To reveal the molecular mechanism leading to replication stress in aneuploid hPSCs, we performed bioinformatics analyses. Expression microarrays of diploid hPSCs (n = 31) were compared to expression microarrays of aneuploid hPSCs (n = 9). The only significant change between these 2 groups was downregulation of the serum response factor (SRF) transcription factor and its targets, the actin genes. Genetic downregulation of SRF or chemical perturbation of the actin cytoskeleton organization in diploid hPSCs recapitulated the findings in aneuploid hPSCs, including the ongoing chromosomal instability. Importantly, SRF overexpression in aneuploid hPSCs resulted in significant rescue of this phenotype. These results suggest that an SRF-dependent mechanism leads to replication stress in hPSCs. SRF regulates the activity of many immediate-early genes and, as implied by its name, its expression is increased by environmental cues like serum stimulation. We showed that serum elimination results in downregulation of SFR in cultured hPSCs.Citation6 Given the deleterious effect of reduced SFR levels on genome stability in hPSCs, and the great therapeutic promise that these cells hold, our study has important implications regarding optimal and safe hPSC culture conditions. Furthermore, PSCs share several similarities with cancer cells, including indefinite self-renewal capacity, aberrant cell cycle checkpoints, and inherent replication stress. Thus, similar mechanisms might account for the ongoing chromosomal instability that allows them to constantly change and adapt. Therefore, studying the role of SRF and actin cytoskeleton organization proteins in cancer, especially CIN positive cancer, is highly important and may shed a new light on chromosomal instability in cancer.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Bester AC, Roniger M, Oren YS, Im MM, Sarni D, Chaoat M, Bensimon A, Zamir G, Shewach DS, Kerem B. Nucleotide deficiency promotes genomic instability in early stages of cancer development. Cell 2011; 145(3):435-46; PMID:21529715; http://dx.doi.org/10.1016/j.cell.2011.03.044
- Burrell RA, McClelland SE, Endesfelder D, Groth P, Weller MC, Shaikh N, Domingo E, Kanu N, Dewhurst SM, Gronroos E, et al. Replication stress links structural and numerical cancer chromosomal instability. Nature 2013; 494(7438):492-6; PMID:23446422; http://dx.doi.org/10.1038/nature11935
- Lamm N, Maoz K, Bester AC, Im MM, Shewach DS, Karni R, Kerem B. Folate levels modulate oncogene-induced replication stress and tumorigenicity. EMBO Mol Med 2015; 7(9):1138-52. Epub 2015/07/23; PMID:26197802; http://dx.doi.org/10.15252/emmm.201404824
- Weissbein U, Benvenisty N, Ben-David U. Quality control: Genome maintenance in pluripotent stem cells. J Cell Biol. 2014; 204(2):153-63. Epub 2014/01/22; PMID:24446481; http://dx.doi.org/10.1083/jcb.201310135
- Ben-David U, Mayshar Y, Benvenisty N. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 2011; 9(2):97-102. Epub 2011/08/06; PMID:21816361; http://dx.doi.org/10.1016/j.stem.2011.06.013
- Lamm N, Ben-David U, Golan-Lev T, Storchova Z, Benvenisty N, Kerem B. Genomic Instability in Human Pluripotent Stem Cells Arises from Replicative Stress and Chromosome Condensation Defects. Cell Stem Cell 2016; 18(2):253-61; PMID:26669899; http://dx.doi.org/10.1016/j.stem.2015.11.003
- Ben-David U, Arad G, Weissbein U, Mandefro B, Maimon A, Golan-Lev T, Narwani K, Clark AT, Andrews PW, Benvenisty N, et al. Aneuploidy induces profound changes in gene expression, proliferation and tumorigenicity of human pluripotent stem cells. Nat Commun 2014; 5:4825; Epub 2014/09/10; PMID:25198699; http://dx.doi.org/10.1038/ncomms5825
- Passerini V, Ozeri-Galai E, de Pagter MS, Donnelly N, Schmalbrock S, Kloosterman WP, Kerem B, Storchová Z. The presence of extra chromosomes leads to genomic instability. Nat Commun 2016;7:10754; PMID:26876972; http://dx.doi.org/10.1038/ncomms10754
- Ahuja AK, Jodkowska K, Teloni F, Bizard AH, Zellweger R, Herrador R, Ortega S, Hickson ID, Altmeyer M, Mendez J, et al. A short G1 phase imposes constitutive replication stress and fork remodelling in mouse embryonic stem cells. Nat Commun 2016; 7:10660; PMID:26876348; http://dx.doi.org/10.1038/ncomms10660
- Ruiz S, Lopez-Contreras AJ, Gabut M, Marion RM, Gutierrez-Martinez P, Bua S, Ramirez O, Olalde I, Rodrigo-Perez S, Li H et al. Limiting replication stress during somatic cell reprogramming reduces genomic instability in induced pluripotent stem cells. Nat Commun 2015; 6:8036. Epub 2015/08/22; PMID:26292731; http://dx.doi.org/10.1038/ncomms9036
