ABSTRACT
The mevalonate pathway results in the prenylation of small GTPases, which are pivotal for oncogenesis and cancer malignancies. However, inhibitors of this pathway, such as statins, have not necessarily produced favorable results in clinical trials. We recently identified properties of statin responders, together with the underlying molecular mechanisms and simple biomarkers to predict these responders.
Abbreviations
| MVP | = | mevalonate pathway |
| RTKs | = | receptor tyrosine kinases |
The mevalonate pathway (MVP) is fundamental for cellular lipid metabolism leading to cholesterol biosynthesis. This pathway also produces farnesyl-pyrophosphate, which leads to the production of molecules such as dolichol, ubiquinone, and geranylgeranyl-pyrophosphate. The rate-limiting enzyme of this pathway, 3-hydroxyl-3-methylglutaryl-CoA (HMG-CoA) reductase, has been recognized as an excellent target for inhibition of the MVP. Mevastatin (also called compactin) was the first natural compound found to inhibit this reductase.Citation1 This was followed by the identification of another natural compound, lovastatin, which was found to be safer than mevastatin in preclinical trials, and its synthetic derivative simvastatin. By the late 1980s lovastatin was demonstrated to be safe and effective for reducing blood levels of cholesterol and low-density lipoprotein in certain patients, such as those with heterozygous familial hypercholesterolaemia; lovastatin was approved by the FDA in 1987.
In 1982, a mutation in the gene encoding RAS small-GTPase was identified as a cause of human cancer. By the early 1990s, many small-GTPases, including other RAS-family and RHO-family members, had been identified as playing important roles in oncogenesis and cancer malignancy. Most of these small-GTPases require lipid modifications such as geranylgeranylation, farnesylation, palmitoylation, and myristoylation for their functions. Geranylgeranylation and farnesylation are categorized as prenylations and occur via MVP, whereas palmitoylation and myristoylation are acylations that occur via fatty acid biosynthesis. The requirement for prenylation of these small-GTPases led researchers to consider inhibition of the MVP as an ideal strategy for the treatment of cancers, as suggested by Brown and Goldstein who first described the MVP.Citation2 Since then, a number of clinical trials with statins, as well as other inhibitors of protein geranylgeranylation and farnesylation, have been conducted. However, contrary to expectations and in vitro results, these clinical trials have produced mixed results in which statins, even in combination with other drugs or with radiation therapy, are not always effective in treating cancers. Most of the small-GTPases that play roles in cancer are expressed ubiquitously and constitutively. These small-GTPases with housekeeping functions might not be meaningful targets of statins for the treatment of cancers. Nonetheless, it is still noteworthy that retrospective studies of large and random populations have indicated that long-term intake of statins tends to reduce the risk of cancer-related mortality,Citation3 although it is not clear whether such effects are primarily due to the inhibition of small-GTPases or instead reflect the improved health conditions of individuals on statin treatment.
We recently identified characteristics that predict possible responders of MVP-based cancer treatment (see ).Citation4 A series of our previous studies have shown that ARF6 and its signaling proteins, namely A Multi-domain ARF GAP Protein 1 (AMAP1, also called ASAP1/DDEF1) and Erythrocyte Membrane Protein Band 4.1 Like 5 (EPB41L5) are frequently overexpressed in different cancers.Citation4-7 Similar to RAS and RHO, ARF6 is a housekeeping small-GTPase, and primarily regulates recycling of plasma membrane components at cell peripheries. However, upon overexpression of ARF6, AMAP1, and EPB41L5, the ARF6-AMAP1-EPB41L5 pathway becomes crucial for the promotion of invasion and metastasis through enhanced integrin recycling and also becomes crucial for drug resistance by yet unidentified mechanisms.Citation4-7 During invasion and metastasis, ARF6 is activated by GEP100, which is recruited to ligand-activated receptor tyrosine kinases (RTKs) such as epidermal growth factor receptor, hepatocyte growth factor receptor (also known as c-MET), and vascular endothelial cell growth factor receptor 2Citation8 or, alternatively, ARF6 is activated by EFA6, which binds to GTP-bound Gα12 that is released from G-protein-coupled receptors activated by lysophosphatidic acid (LPA-GPCR).Citation7 ARF6 is acylated but not prenylated. Nonetheless, we demonstrated that the MVP is essential for the activation of ARF6.Citation4 In this process, MVP is essential for the geranylgeranylation of RAB11b, which mediates the intracellular trafficking of ARF6 to the plasma membrane to be activated by RTKs. Transforming growth factor β1 (TGFβ1), a risk factor for breast cancer, also requires MVP to activate ARF6, though which TGFβ1 trans-activates c-MET.Citation4 Moreover, consistent with a report by C. Prives's research group that gain-of-function mutants of p53 (a protein product of TP53, best known as p53) upregulate MVP (see ref. Citation4), mutant p53s are crucial for ARF6 activation.Citation4 Therefore, MVP appears to be crucial for the promotion of invasion, metastasis, and drug resistance of cancer cells using the overexpressed ARF6 pathway that is activated by RTKs. Remarkably, moreover, our in vitro experiments indicated that the presence of statins at approximately 1 nM is sufficient to improve the sensitivities of breast cancer cells to different drugs by approximately 1,000 fold. Inhibition of MVP, on the other hand, is ineffective if the cancer cells do not overexpress components of the ARF6-based pathway.Citation4
Figure 1. Mechanisms of tumor responsiveness to statins. Overexpression of the ARF6-AMAP1-EPB41L5 pathway critically promotes invasion, metastasis, and drug resistance of cancers. ARF6 requires RAB11b to be activated at the plasma membrane by receptor tyrosine kinases (indicated as Receptors), whereas the mevalonate pathway is essential to RAB11b function by mediating its geranylgeranylation by geranylgeranyl transferase-II. Mutant p53 proteins, which are known to activate the mevalonate pathway, hence become driving forces to promote ARF6-based malignancies. Possible involvement of estrogen stimulation in ARF6 activation needs further experimental scrutiny. Proposed surrogate markers predictive of statin responders by their combinations are indicated (*; combinations are indicated by the same colors). Abbreviations: AMAP1, A Multi-domain ARF GAP Protein 1; EPB41L5, Erythrocyte Membrane Protein Band 4.1 Like 5; GGT-II, geranylgeranyl transferase-II; HMGCR, HMG-CoA reductase; MVP, mevalonate pathway.
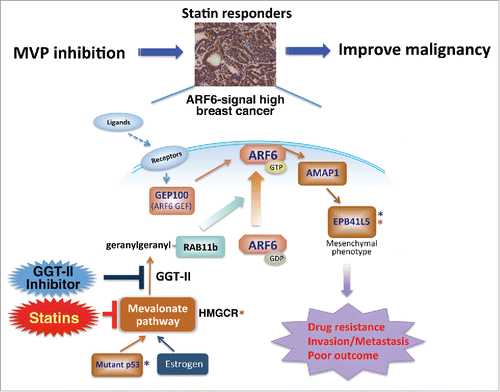
Statistically, overexpression of every component of the ARF6-based pathway in primary breast cancer tightly correlates with poor overall survival of patients (P = 0.04189; to be published elsewhere). For the clinical application of our results, we have proposed 2 surrogate markers that appear to accurately predict breast cancer patients who are statin responders: (1) co-overexpression of EPB41L5 with HMGCR (encoding HMG-CoA reductase) (P = 0.0053; ref. Citation4), and (2) overexpression of EPB41L5 in the presence of missense mutations of TP53 (many of which may encode gain-of-function mutant p53s) (P = 0.00003473; to be published elsewhere). In this regard, it is interesting to note that EPB41L5 is a mesenchymal-specific gene, and cancer cells (which are of epithelial origin) do not express it unless they undergo mesenchymal transformation.Citation4,7 Moreover, these predictions were based on mRNA levels, and it should be noted that protein levels of ARF6 and AMAP1 are primarily determined post-transcriptionally.Citation6 Protein expression levels of EPB41L5 correlated well with its mRNA levels. We have yet to perform immunohistochemical analyses of every component of the ARF6-based pathway in breast cancer, but we have done so in renal cancer and shown that immunohistochemical detection of these components is a powerful tool for predicting the prognosis and overall survival of patients.Citation7
Breast conservative therapy has become very popular in the treatment of breast cancer. Our previous study, in which we examined the expression of AMAP1 and GEP100 by immunohistochemistry, demonstrated that co-overexpression of these 2 proteins exhibits a tight correlation with rapid local recurrence after breast conservative therapy, indicating that the ARF6-based pathway plays a central role in recurrence.Citation9 In accordance with this result, a recent report showed that the recurrence rates of breast cancer can be reduced by statins.Citation10 Other types of cancers, such as lung adenocarcinomas and head and neck squamous cell carcinomas, also overexpress the ARF6-based pathway (see refs. Citation4, 7). Thus, it will be interesting to examine whether statins are also effective for the treatment of these types of cancer. Moreover, we have yet to clarify whether MVP is also involved in the activation of ARF6 by LPA-GPCR signaling.
Cellular activities of MVP, particularly the activity of the allosteric enzyme HMG-CoA reductase, are controlled by various factors. These activities therefore change dynamically depending on the conditions of individuals, including cholesterol intake and plasma concentrations of steroid hormones such as estrogen, and also on oncogenic mutations of TP53. We found that ARF6 becomes highly activated in mammary epithelial cells even in the presence of normal p53 (our unpublished results). Thus, the physical condition of patients, including their nutritional status, should be taken into account when the usefulness of statins is re-evaluated using ARF6-based biomarkers predictive of statin responders.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- Endo A, Kuroda M, Tsujita Y. ML-236A, ML-236B, and ML-236C, new inhibitors of cholesterogenesis produced by Penicillium citrinium. J Antibiot 1976; 29:1346-1348; PMID:1010803; http://dx.doi.org/10.7164/antibiotics.29.1346
- Goldstein JL, Brown MS. 1990. Regulation of the mevalonate pathway. Nature 1990; 343:425-430; PMID:1967820; http://dx.doi.org/10.1038/343425a0
- Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med 2012; 367:1792-1802; PMID:23134381; http://dx.doi.org/10.1056/NEJMoa1201735
- Hashimoto A, Oikawa T, Hashimoto S, Sugino H, Yoshikawa A, Otsuka Y, Handa H, Onodera Y, Nam JM, Oneyama C, et al. P53- and mevalonate pathway-driven malignancies require Arf6 for metastasis and drug resistance. J Cell Biol 2016; 213:81-95; PMID:27044891; http://dx.doi.org/10.1083/jcb.201510002
- Sabe H. Requirement for Arf6 in cell adhesion, migration, and cancer cell invasion. J Biochem 2003; 134:485-489; PMID:14607973; http://dx.doi.org/10.1093/jb/mvg181
- Sabe H, Hashimoto S, Morishige M, Ogawa E, Hashimoto A, Nam JM, Miura K, Yano H, Onodera Y. The EGFR-GEP100-Arf6-AMAP1 signaling pathway specific to breast cancer invasion and metastasis. Traffic 2009; 10: 982-993; PMID:19416474; http://dx.doi.org/10.1111/j.1600-0854.2009.00917.x
- Hashimoto S, Mikami S, Sugino H, Yoshikawa A, Hashimoto A, Onodera Y, Furukawa S, Handa H, Oikawa T, Okada Y, et al. Lysophosphatidic acid activates Arf6 to promote the mesenchymal malignancy of renal cancer. Nat Commun 2016; 7:10656; PMID:26854204; http://dx.doi.org/10.1038/ncomms10656
- Morishige M, Hashimoto S, Ogawa E, Toda Y, Kotani H, Hirose M, Wei S, Hashimoto A, Yamada A, Yano H, et al. GEP100 links EGFR signaling to Arf6 activation to induce breast cancer invasion. Nat Cell Biol. 2008; 10:85-92; PMID:18084281; http://dx.doi.org/10.1038/ncb1672
- Kinoshita R, Nam JM, Ito YM, Hatanaka KC, Hashimoto A, Handa H, Otsuka Y, Hashimoto S, Onodera Y, Hosoda M, et al. Co-overexpression of GEP100 and AMAP1 proteins correlates with rapid local recurrence after breast conservative therapy. PLoS ONE 2013; 8:e76791; PMID:24116160; http://dx.doi.org/10.1371/journal.pone.0076791
- Ahern TP, Lash TL, Damkier P, Christiansen PM, Cronin-Fenton DP. Statins and breast cancer prognosis: evidence and opportunities. Lancet Oncol 2014; 15:e461-8; PMID:25186049; http://dx.doi.org/10.1016/S1470-2045(14)70119-6
