ABSTRACT
We have recently demonstrated that protein kinase Cι (PKCι) promotes a stem-like, tumor-initiating cell phenotype in KRAS-driven lung adenocarcinoma by activating a novel ELF3-NOTCH3 signaling axis.Citation1 Combined PKCι and NOTCH inhibition was identified as a novel strategy for the treatment of KRAS-driven lung adenocarcinoma.
Protein Kinase Cι (PKCι) is an oncogene that promotes tumorigenesis in multiple human tumor types in vitro and in vivo.Citation2 PKCι is genetically altered and overexpressed in many tumor types, including gliomas, myelogenous leukemias, and cancers of the lung, colon, pancreas, ovary, and breast.Citation2 We recently demonstrated that PKCι is overexpressed in lung squamous cell carcinoma (LSCC) as a result of tumor-specific copy number gains (CNGs) of the PRKCI gene encoding PKCι. PRKCI resides on chromosome 3q26, which is one of the most frequently amplified genomic regions in human cancers.Citation3 PRKCI CNGs occur in ∼70% of LSCCs where they drive PKCι expression, transformed growth, and invasion.Citation3 In LSCC tumors PRKCI is co-amplified with SOX2, a master regulator of stemness that also resides on chromosome 3q26.Citation3 PRKCI and SOX2 cooperate to drive a stem-like tumor-initiating cell (TIC) phenotype by inducing expression of hedgehog acyl transferase (HHAT), which regulates hedgehog (Hh) ligand production. PKCι phosphorylates SOX2 to regulate its occupancy on the HHAT promoter, HHAT expression, and maintenance of a LSCC stem-like phenotype.Citation3 Thus, 3q26 CNGs drive coordinated expression of 2 genes on 3q26, PRKCI and SOX2, that are genetically, biochemically and functionally linked to establishment of a stem-like LSCC TIC phenotype. Interestingly, we identified the guanine nucleotide exchange factor (GEF) ECT2 as an oncogenic protein that binds PKCι.Citation4 ECT2 also resides on chromosome 3q26 and is coordinately amplified and overexpressed with PRKCI and SOX2 in LSCC.Citation5 ECT2 binds an oncogenic PKCι-Par6 complex and is phosphorylated by PKCι to activate the small GTPase RAC1. RAC1 in turn activates a PKCι-PAR6-ECT2-RAC1-MEK-ERK signaling cascade that drives LSCC transformed growth and invasion.Citation5 Our data suggest that chromosome 3q26 amplification functions as an “OncCassette” that drives transformation through coordinated overexpression of multiple genes that drive oncogenic signaling cascades centered on PKCι (). PKCι-SOX2-HHAT signaling establishes and maintains a LSCC TIC phenotype, whereas PKCι-PAR6-ECT2-MEK-ERK signaling drives LSCC cell growth and invasion.
Figure 1. PKCι drives both common and lineage-specific oncogenic pathways in lung cancer. In KRAS-driven lung adenocarcinoma (KRAS-driven LADC) PKCι acts as a critical downstream KRAS effector, activating a novel PKCι-ELF3-NOTCH3 signaling axis that establishes and maintains a KRAS-driven LADC TIC phenotype. In lung squamous cell carcinoma (LSCC) harboring 3q26 amplification, the 3q26 genes PRKCI, SOX2, and ECT2 are coordinately amplified and overexpressed, leading to lineage-specific activation of a PKCι-SOX2-HHAT signaling axis. This pathway drives a Hedgehog-dependent LSCC tumor-initiating cell (TIC) phenotype characterized by highly aggressive tumorigenic and invasive behavior and enhanced survival and chemoresistance. The PKCι gene target GLI1 is a major effector of this pathway. PKCι also activates a PKCι-ECT2-RAC1-MEK-ERK signaling pathway that drives transformed growth and invasion of both KRAS LADC and LSCC cells. The PKCι target matrix metalloproteinase 10 (MMP10) is a major effector of this pathway. The PKCι inhibitor auranofin exhibits antitumor activity against both LSCC and KRAS-driven LADC, and exhibits lineage-specific synergistic activity when combined with a NOTCH inhibitor in KRAS-driven LADC and a SMO inhibitor in LSCC.
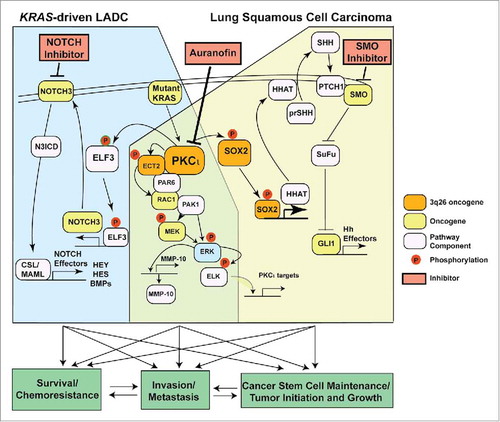
Interestingly, PKCι is also overexpressed in LADC tumors harboring oncogenic KRAS mutations (KRAS-driven LADC) despite the absence of PRKCI CNGs.Citation6 PKCι drives transformed growth of mutant KRAS LADC cells in vitro and lung tumorigenesis in LSL-Kras mice in vivo.Citation7 As in LSCC, PKCι promotes LADC proliferation and invasion by activating the PKCι-PAR6-ECT2-RAC1-PAK-MEK-ERK signaling cascade.Citation5
It was unknown whether PKCι maintains a TIC phenotype in KRAS-driven LADC; however, our recent studies resolved this issue.Citation1 Specifically, we showed that PKCι establishes and maintains a KRAS-driven LADC TIC phenotype. Interestingly, KRAS-driven LADC TICs do not exhibit Hh-dependent growth or activate PKCι-SOX2-HHAT signaling;Citation3 rather, PKCι maintains KRAS-driven LADC TICs through a distinct PKCι-ELF3-NOTCH3 signaling axis ().Citation1 PKCι controls expression of NOTCH3, a key driver of stemness in KRAS-driven LADC, by phosphorylating the ELF3 transcription factor and driving ELF3 occupancy on the NOTCH3 promoter.Citation1 We found that PKCι-ELF3-NOTCH3 signaling controls LADC TIC fate by controlling asymmetric cell division,Citation1 a fundamental process necessary for tumor initiation, maintenance, and progression. These findings may have important therapeutic implications. We identified the antirheumatoid compounds aurothiomalate (ATM) and auranofin (ANF) as selective PKCι inhibitors exhibiting good antitumor activity;Citation2 our early clinical trials suggest that these agents may be useful in treating LSCC and KRAS-driven LADC (and ovarian serous carcinoma).Citation8,9 In this regard, ANF can be effectively combined with a second agent targeting one of the lineage-restricted oncogenic PKCι signaling pathways outlined here. Thus, combined PKCι and SMO inhibition is synergistic against LSCC,Citation10 whereas combined ANF and NOTCH inhibition shows synergy against KRAS-driven LADC.Citation1 These targeted drug combinations may provide novel therapeutic approaches to more effectively treat LSCC and KRAS-driven LADC, two common and deadly cancers with few therapeutic options.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The work described herein was supported by grants from the National Institutes of Health (R01 CA081436-17 and R21 CA151250-02 to APF; R01 CA14090-05 to NRM; and R21 CA204938-01 (VJ); the James and Esther King Biomedical Research Program (1KG-05-33971) to APF, the Mayo Clinic Center for Individualized Medicine (CIM) to APF; a National Institutes of Health Research Supplement to Promote Diversity in Health-related Research Award from the National Cancer Institute to VJ, and the George Haub Family Career Development Award (VJ). APF is the Monica Flynn Jacoby Professor of Cancer Research. SAA is the recipient of the Edward C. Kendall Fellowship in Biochemistry from the Mayo Clinic Graduate School.
Additional information
Funding
References
- Ali SA, Justilien V, Jamieson L, Murray NR, Fields AP. Protein Kinase Ciota Drives a NOTCH3-dependent Stem-like Phenotype in Mutant KRAS Lung Adenocarcinoma. Cancer Cell 2016; 29:367-78; PMID:26977885; https://doi.org/10.1016/j.ccell.2016.02.012
- Parker PJ, Justilien V, Riou P, Linch M, Fields AP. Atypical Protein Kinase Ciota as a human oncogene and therapeutic target. Biochem Pharmacol 2014; 88:1-11; PMID:24231509; https://doi.org/10.1016/j.bcp.2013.10.023
- Justilien V, Walsh MP, Ali SA, Thompson EA, Murray NR, Fields AP. The PRKCI and SOX2 oncogenes are coamplified and cooperate to activate Hedgehog signaling in lung squamous cell carcinoma. Cancer Cell 2014; 25:139-51; PMID:24525231; https://doi.org/10.1016/j.ccr.2014.01.008
- Justilien V, Fields AP. Ect2 links the PKCiota-Par6alpha complex to Rac1 activation and cellular transformation. Oncogene 2009; 28: 3597-607; PMID:19617897; https://doi.org/10.1038/onc.2009.217
- Justilien V, Jameison L, Der CJ, Rossman KL, Fields AP. Oncogenic activity of Ect2 is regulated through protein kinase C iota-mediated phosphorylation. J Biol Chem 2011; 286: 8149-57; PMID:21189248; https://doi.org/10.1074/jbc.M110.196113
- Regala RP, Weems C, Jamieson L, Khoor A, Edell ES, Lohse CM et al. Atypical protein kinase C iota is an oncogene in human non-small cell lung cancer. Cancer Res 2005; 65: 8905-11; PMID:16204062; https://doi.org/10.1158/0008-5472.CAN-05-2372
- Regala RP, Davis RK, Kunz A, Khoor A, Leitges M, Fields AP. Atypical protein kinase C{iota} is required for bronchioalveolar stem cell expansion and lung tumorigenesis. Cancer Res 2009; 69: 7603-11; PMID:19738040; https://doi.org/10.1158/0008-5472.CAN-09-2066
- Jatoi A, Radecki Breitkopf C, Foster NR, Block MS, Grudem M, Wahner Hendrickson A et al. A Mixed-Methods Feasibility Trial of Protein Kinase C Iota Inhibition with Auranofin in Asymptomatic Ovarian Cancer Patients. Oncology 2014; 88: 208-13; PMID:25502607; https://doi.org/10.1159/000369257
- Mansfield AS, Fields AP, Jatoi A, Qi Y, Adjei AA, Erlichman C et al. Phase I dose escalation study of the PKCiota inhibitor aurothiomalate for advanced non-small-cell lung cancer, ovarian cancer, and pancreatic cancer. Anticancer Drugs 2013; 24: 1079-83; PMID:23962904; https://doi.org/10.1097/CAD.0000000000000009
- Justilien V, Fields AP. Molecular pathways: novel approaches for improved therapeutic targeting of hedgehog signaling in cancer stem cells. Clin Cancer Res 2015; 21: 505-13; PMID:25646180; https://doi.org/10.1158/1078-0432.CCR-14-0507
