ABSTRACT
Our recent studies determined molecular interactions between genes in the ubiquitin-proteasome pathways and cancer cell metabolism. Ubiquitin-specific peptidase 13 (USP13) specifically deubiquitinates and thus upregulates ATP citrate lyase and oxoglutarate dehydrogenase that drive ovarian cancer metabolism. These findings may lead to the development of USP13 inhibitors and new-targeted therapies in ovarian cancers.
High-grade serous ovarian cancer (HGSC) is the most lethal cause of gynecological cancer deaths which accounts for 70–80% of ovarian cancer (OVCA) deaths. Overall survival for patients with HGSC has not much improved in the past 30 y, and standard therapy has not advanced beyond platinum-based combination chemotherapy.Citation1 Targeting metabolic dependencies of cancer cells has been suggested as a selective anticancer strategy.Citation2 For example, high-invasive ovarian cancer cells are markedly dependent on glutamine to meet the requirements of abundant building blocks and energy for their cell growth and division. Indeed, glutamine catabolism correlates with poor survival in ovarian cancer patients.Citation3 Further, upregulated glutamine anabolism in stromal cells can support glutamine catabolism of cancer cells.Citation4 Global analysis of genomic alteration across cancers has shown that ovarian cancers have high copy number changes with low frequency of recurrent mutations. Consistently, mutation events on metabolic enzymes are relatively infrequent in ovarian cancer. Therefore, we assume that posttranslational modifications such as ubiquitination play a central role in regulating protein stability and activity of metabolic enzymes or signaling pathways that hardwire metabolism to tumorigenesis. In our recent study, we conducted bioinformatic analyses of ovarian cancer databases and metabolic tracing experiments and determined the molecular interactions between genes in the ubiquitin-proteasome pathways and ancer cell metabolism ().Citation5
Figure 1. USP13 amplification drives ovarian cancer metabolism. Ubiquitin Specific Peptidase 13 (USP13) gene is frequently (29.3%) amplified in ovarian cancer. As a deubiquitinase, USP13 enhances metabolic efficiency in ovarian cancer cells by directly regulating multiple metabolic pathways. USP13 deubiquitinates and upregulates two key metabolic enzymes, oxoglutarate dehydrogenase (OGDH) and ATP citrate lyase (ACLY), in glutaminolysis and lipid synthesis. Inhibiting USP13 dramatically suppresses ovarian tumor progression and sensitizes tumor cells to the treatment of PI3K/AKT inhibitors. CNV, Copy number variation.
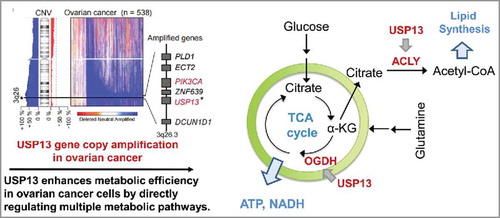
The ubiquitin-proteasome system is the central system for controlling protein degradation and regulatory function. Ubiquitin-specific proteases (USPs) are the largest family of deubiquitinates that catalyze the removal of ubiquitin moieties from specific target proteins regulating distinct cellular processes.Citation6 Genomic analyses of ovarian cancer revealed that USP13 gene is amplified in 29.3% of HGSC. Interestingly, USP13 gene is adjacent to phosphatidylinositol-3-kinase catalytic subunit, α-isoform (PIK3CA) in the amplicon across chromosome 3q26.2–3q26.3. Expression of USP13 is markedly increased in advanced ovarian tumors and significantly correlated with poor survival of patients with ovarian cancers. In particular, we observed that depletion of USP13 dramatically suppressed the proliferation of USP13—highly expressed ovarian cancer cell lines—as well as in vivo tumor growth and metastasis. It suggests that OVCA cells harboring USP13 amplication have more dependency on USP13 level. Further, inhibition of USP13 leads to selective supersession of OVCA cell proliferation and tumor growth, and that the role of USP13 in oncogenesis is context dependent.
ATP citrate lyase (ACLY) and oxoglutarate dehydrogenase (OGDH) were identified as deubiquitinase targets of USP13. USP13 removes K48-associted ubiquitination on ACLY and OGDH, thus upregulates the stability of ACLY and OGDH. As a subunit of α-ketoglutarate dehydrogenase (KGDH) complex, OGDH is a rate-limiting component for the overall conversion of α-ketoglutarate (α-KG) to succinyl-CoA and CO2. Glutamine enters the tricarboxylic acid (TCA) cycles as α-KG via the KGDH complex to replenish TCA cycle intermediates and sustain anabolic processes in the cancer cells.Citation7 Recent studies reported that inhibition of OGDH leads to buildup of lactic acid and suppresses cell growth. Furthermore, oxidation of α-KG is required for reductive carboxylation in cancer cells with mitochondrial defects.Citation8 ACLY is a critical enzyme involved in generating acetyl-CoA, a building block for de novo lipid synthesis. ACLY is often upregulated or activated in human cancers, including lung, prostate, bladder, breast, liver, stomach, and colon tumors.Citation9
To determine the impact of USP13 on cancer cell metabolism, we performed the metabolic tracing analysis. We found that depletion of USP13 inhibits glutaminolysis and induces mitochondrial dysfunction in ovarian cancer cells. We also found that USP13 knockdown cells decreased glutamine-driven reductive carboxylation and lipid synthesis. These findings demonstrate that inhibition of USP13 simultaneously suppresses glutamate anaplerosis to replenish the TCA cycle and the generation of acetyl-CoA, a vital building block for de novo biosynthesis of fatty acids. In addition, we determined that ACLY and OGDH are key metabolic enzymes that drive ovarian cancer cell proliferation. Overexpression either or both of ACLY and OGDH dramatically rescued the proliferation of USP13 knockdown ovarian cancer cell lines. In vivo tumor growth was also greatly rescued by ACLY and OGDH overexpression.
In summary, our studies suggest that USP13 amplification is likely an important driver in ovarian tumor progression, and targeting of USP13 will be a novel therapeutic approach for ovarian cancers harboring such common genomic alterations. Our data suggest that amplification of USP13 allows OVCA cells to rely on glutamine anaplerosis to replenish the TCA cycle with metabolic intermediates, and USP13 knockdown represses mitochondrial function. In particular, we also found that inhibiting USP13 sensitizes OVCA cells to the treatment of AKT inhibitor, suggesting a synergistic role of USP13 in the PI3K/AKT-induced tumorigenesis. Since the PI3K/AKT/mTOR pathway is frequently altered in OVCA,Citation10 amplification of USP13 may be a part of mechanism for intrinsic resistance of PI3K/AKT inhibitors in the treatment of OVCA. Our study provides a potential therapeutic strategy in which targeting USP13 blocks biosynthesis of metabolic intermediates and lipids thereby simultaneously inducing energy stress and cell death. Combined treatment of PI3K/AKT inhibitor with USP13 inhibitor can be a promising targeting strategy to overcome the resistance of PI3K/AKT inhibitors.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
This work was supported by grants to X.L. from the NIH (CA185742 and CA203737) and to C.H. from the NIH (CA197487).
References
- Bowtell DD, Bohm S, Ahmed AA, Aspuria PJ, Bast RC, Jr, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et al. Rethinking ovarian cancer II: reducing mortality from high-grade serous ovarian cancer. Nat Rev Cancer 2015; 15(11):668-79; PMID:26493647; https://doi.org/10.1038/nrc4019
- Vander Heiden MG. Targeting cancer metabolism: a therapeutic window opens. Nat Rev Drug Discov 2011; 10(9):671-84; PMID:21878982; https://doi.org/10.1038/nrd3504
- Yang L, Moss T, Mangala LS, Marini J, Zhao H, Wahlig S, Armaiz-Pena G, Jiang D, Achreja A, Win J, et al. Metabolic shifts toward glutamine regulate tumor growth, invasion and bioenergetics in ovarian cancer. Mol Syst Biol 2014; 10:728; PMID:24799285; https://doi.org/10.1002/msb.20134892
- Yang L, Achreja A, Yeung TL, Mangala LS, Jiang D, Han C, Baddour J, Marini JC, Ni J, Nakahara R, et al. Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab 2016; 24(5):685-700; PMID:27829138; https://doi.org/10.1016/j.cmet.2016.10.011
- Han C, Yang L, Choi HH, Baddour J, Achreja A, Liu Y, Li Y, Li J, Wan G, Huang C, et al. Amplification of USP13 drives ovarian cancer metabolism. Nat Commun 2016; 7:13525; PMID:27892457; https://doi.org/10.1038/ncomms13525
- D'Arcy P, Linder S. Proteasome deubiquitinases as novel targets for cancer therapy. Int J Biochem Cell Biol 2012; 44(11):1729-38; PMID:22819849; https://doi.org/10.1016/j.biocel.2012.07.011
- Ahn CS, Metallo CM. Mitochondria as biosynthetic factories for cancer proliferation. Cancer Metab 2015; 3(1):1; PMID:25621173; https://doi.org/10.1186/s40170-015-0128-2
- Mullen AR, Hu Z, Shi X, Jiang L, Boroughs LK, Kovacs Z, Boriack R, Rakheja D, Sullivan LB, Linehan WM, et al. Oxidation of alpha-ketoglutarate is required for reductive carboxylation in cancer cells with mitochondrial defects. Cell Rep 2014; 7(5):1679-90; PMID:24857658; https://doi.org/10.1016/j.celrep.2014.04.037
- Chypre M, Zaidi N, Smans K. ATP-citrate lyase: a mini-review. Biochem Biophys Res Commun 2012; 422(1):1-4; PMID:22575446; https://doi.org/10.1016/j.bbrc.2012.04.144
- Patch AM, Christie EL, Etemadmoghadam D, Garsed DW, George J, Fereday S, Nones K, Cowin P, Alsop K, Bailey PJ, et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015; 521(7553):489-94; PMID:26017449; https://doi.org/10.1038/nature14410
