ABSTRACT
Cyclin-dependent kinase 4 (CDK4) is a positive regulator of cell cycle progression, however, there is growing evidence demonstrating that its function exceeds the control of cell division. Here we show that CDK4 is an important regulator of cellular substrate utilization through direct inhibition of the metabolic regulator AMPK (AMP-activated protein kinase).
Metabolic adaptation has been recently found in the spotlight for research due to its importance for numerous physiological processes, like insulin responsiveness by liver and white adipose tissue, cold responsiveness by brown adipose tissue, exercise performance by muscle, and T cell response to an infection. Metabolic remodeling is also a hallmark of cancer cells,Citation1 and characterizes pathological states, like insulin resistance and aging.Citation2
In recent years, we and others have discovered key roles for cell cycle regulators in the control of metabolic processes in physiological and pathological conditions. Amongst these, the cyclin dependent kinase 4 (CDK4) is now recognized as not only a coordinator between cellular metabolism and cell cycle progression,Citation3 but also a key metabolic regulator in differentiated, non-proliferating cells.Citation4 Indeed, CDK4 has been shown to promote adipose tissue differentiation by phosphorylating and activating Peroxisome proliferator-activated receptor gamma (PPAR-γ),Citation5 to maintain a positive feedback loop in the insulin signaling pathway by phosphorylating Insulin Receptor Substrate 2 (IRS2)Citation6 and to suppress hepatic glucose production by phosphorylating and activating general control non-repressed protein 5 (GCN5).Citation7 CDK4 has also been shown to participate in the control of metabolism via the canonical CDK4-pRB-E2F1 pathway, involving the retinoblastoma protein (RB1, best known as pRB) and the E2F1 transcription factor.Citation3 All these evidence suggest that CDK4 promotes anabolic processes. Interestingly, by characterizing substrate utilization in mouse embryonic fibroblasts (MEFs) lacking CDK4 (Cdk4−/−) or expressing a hyperactive version of the protein (Cdk4R24C/R24C), we determined that CDK4 promotes anaerobic glycolysis, while preventing fatty acid oxidation (FAO). This led to our discovery of a novel, unexpected function for CDK4, in phosphorylating and repressing the α2 subunit of the major regulator of oxidative metabolism AMPK (AMP-activated protein kinase).Citation8 The use of chemical inhibitors of CDK4 (LY2835219) drives increased FAO in MEFs, as well as in muscle cells, in an AMPK dependent manner. This finding was further supported by the fact that AMPKα2 mutants that cannot be phosphorylated by CDK4 have increased capacity to promote FAO, when compared to their wild type AMPKα2 counterpart.Citation9
Given that muscle highly express the α2 subunit of AMPK and responds to exercise by down regulating CDK activity and concomitantly increasing AMPK activity and FAO,Citation10 we set out to investigate a potential role for the FAO regulation by CDK4 in muscle function. Interestingly, mice lacking CDK4 in all tissues except in pancreatic beta cells, show increased exercise capacity and decreased respiratory exchange ratio (RER), suggesting higher levels of oxidative metabolism. The treatment of wild type mice with LY2835219 led to a similar increase in exercise capacity and to a similar decrease of RER. This phenotype was dependent on the activity of AMPK in muscle.Citation9
Overall, we believe that CDK4 can rapidly modulate metabolism in proliferating and differentiated cells, as well as at a whole body level. Metabolic pathways, including the AMPK and insulin/insulin-like growth factor 1 (IGF-1) signaling pathways are increasingly becoming druggable targets for anti-tumor therapies. Therefore, the finding that CDK4 promotes proliferation, maintains the insulin signaling pathway, and therefore lipid synthesis, while repressing FAO, is of great interest for translational research given that three different CDK4/6 inhibitors PD0332991 (palbociclib), LY-2835219 (abemaciclib) and LEE011 (ribociclib) are currently approved for the treatment of breast cancer or are currently in advanced stages of clinical trials for certain cancers.
In a broader sense, our study underscores the necessity for cells and whole organisms to establish integrated responses that will ensure a proper metabolic response to external cues. For instance, in the case of cell cycle, mitogen response needs to be coupled to a concerted program to fulfill both the necessity for important energy stores, as well as the requirement for biosynthetic intermediates for nucleic acids, proteins and lipids. This can be orchestrated in a timely manner by using cell cycle regulators to integrate extracellular signals (i.e. mitogens) and regulate signaling pathways in a coordinated manner. At the level of the whole organism, such metabolic master regulators could also ensure a proper metabolic adaptation from the tissues to external stimuli, such as exercise, diet and nutrition.
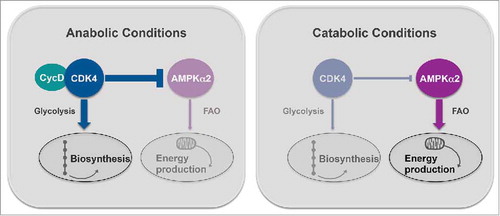
Our recent study places CDK4 as one of such metabolic regulators, which has the ability to promote anabolism, while directly repressing catabolism by inhibiting AMPK (). Our findings indicate that therapeutic strategies targeting CDK4 may have unforeseen beneficial effects, due to the metabolic functions of this cell cycle regulator in pathologies ranging from metabolic disorders to cancer.
Figure 1. CDK4 functions as a cellular sensor of energy status. In anabolic conditions, cyclin-dependent kinase 4 (CDK4) is bound to D-type cyclins (CycD) and therefore, active. In these conditions, it promotes glycolysis as well as biosynthetic pathways, while repressing fatty acid oxidation (FAO) via the inhibition of the α2 subunit of the AMP-activated protein kinase (AMPKα2). On the other hand, when energy levels are low, CDK4 is inactive. Hence, ATP producing processes, like FAO, are triggered in order to restore cellular energy homeostasis and respond to energy stress.
Acknowledgments
We thank Albert Giralt for his critical reading of this manuscript.
Funding
This work was supported by the Swiss National Science Foundation.
References
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi:10.1016/j.cell.2011.02.013
- Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–217. doi:10.1016/j.cell.2013.05.039
- Salazar-Roa M, Malumbres M. Fueling the Cell Division Cycle. Trends Cell Biol. 2017;27:69–81. doi:10.1016/j.tcb.2016.08.009
- Lopez-Mejia IC, Fajas L. Cell cycle regulation of mitochondrial function. Curr Opin Cell Biol. 2015;33:19–25. doi:10.1016/j.ceb.2014.10.006
- Abella A, Dubus P, Malumbres M, Rane SG, Kiyokawa H, Sicard A, Vignon F, Langin D, Barbacid M, Fajas L. Cdk4 promotes adipogenesis through PPARgamma activation. Cell Metab. 2005;2:239–49.doi:10.1016/j.cmet.2005.09.003
- Lagarrigue S, Lopez-Mejia IC, Denechaud PD, Escoté X, Castillo-Armengol J, Jimenez V, Chavey C, Giralt A, Lai Q, Zhang L, et al. CDK4 is an essential insulin effector in adipocytes. J Clin Invest. 2016;126:335–48. doi:10.1172/JCI81480
- Lee Y, Dominy JE, Choi YJ, Jurczak M, Tolliday N, Camporez JP, Chim H, Lim JH, Ruan HB, Yang X, et al. Cyclin D1-Cdk4 controls glucose metabolism independently of cell cycle progression. Nature. 2014;510:547–51. doi:10.1038/nature13267
- Hardie DG, Schaffer BE, Brunet A. AMPK: An Energy-Sensing Pathway with Multiple Inputs and Outputs. Trends Cell Biol. 2016;26:190–201. doi:10.1016/j.tcb.2015.10.013
- Lopez-Mejia IC, Lagarrigue S, Giralt A, Martinez-Carreres L, Zanou N, Denechaud PD, Castillo-Armengol J, Chavey C, Orpinell M, Delacuisine B, et al. CDK4 Phosphorylates AMPKalpha2 to Inhibit Its Activity and Repress Fatty Acid Oxidation. Mol Cell. 2017;68;336–49 e336. doi:10.1016/j.molcel.2017.09.034
- Hoffman NJ, Parker BL, Chaudhuri R, Fisher-Wellman KH, Kleinert M, Humphrey SJ, Yang P, Holliday M, Trefely S, Fazakerley DJ, et al. Global Phosphoproteomic Analysis of Human Skeletal Muscle Reveals a Network of Exercise-Regulated Kinases and AMPK Substrates. Cell Metab. 2015;22:922–35. doi:10.1016/j.cmet.2015.09.001
