ABSTRACT
Non-alcoholic fatty liver disease (NAFLD) is the most common chronic liver disease and is the major risk factor leading to hepatocellular carcinoma (HCC). Cisd2 haploinsufficiency in mice causes NAFLD by disrupting Ca2+ homeostasis, indicating that CISD2 is a molecular target for the treatment of NAFLD and the prevention of HCC.
NAFLD is a major risk factor for HCC. Hepatocellular carcinoma (HCC) is the second most common cause of cancer-associated death worldwide. Recent epidemiological studies have indicated that non-alcoholic fatty liver disease (NAFLD) has become the fastest growing risk factor leading to HCC. NAFLD is the major cause of chronic liver disease and can progress to its more severe form, nonalcoholic steatohepatitis (NASH), which is characterized by necro-inflammation and fibrosis. A number of different pathways/mechanisms, including interplay between oxidative stress, inflammatory cytokines and endoplasmic reticulum (ER) stress, Citation1 have been proposed to explain how NAFLD promotes hepatocarcinogenesis. However, the molecular mechanism upstream to the development of NAFLD remains unclear. A comprehensive study of the mechanistic links leading from normal liver to NAFLD, thence to NASH, and to finally to HCC, will help with the development of new therapeutic strategies for the treatment NAFLD and thus the prevention of HCC.
Abnormal ER Ca2+ homeostasis leads to ER stress and NAFLD. ER is critical to maintaining Ca2+ homeostasis, protein folding, and lipid synthesis. Increased ER stress is one of the key factors that cause liver disease. Specifically, Ca2+ homeostasis in hepatocytes is maintained by appropriately functioning sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2 isoform b (SERCA2b) and inositol 1,4,5-trisphosphate receptor (IP3R), which, respectively, take up and release Ca2+ in response to metabolic signaling. In obesity-related NAFLD, Serca2b protein levels and activity are significantly down-regulated. Conversely, in obese mice, overexpression of Serca2b improves the NAFLD/NASH phenotypes by preventing palmitate-induced ER stress and hepatic cell death. Citation2 These findings reveal that aberrant ER Ca2+ homeostasis can result in ER stress, which then leads to abnormal liver metabolism, causing NAFLD/NASH.
Cisd2 modulates intracellular Ca2+ homeostasis and normal metabolism by regulating liver Serca2b activity. CDGSH iron sulfur domain 2 (CISD2) is the causative gene of Wolfram syndrome 2 and is crucial for maintaining a healthy lifespan in mammals. Cisd2 protein is localized in the ER, the mitochondrial outer membrane and the mitochondria-associated ER membrane. Several studies have indicated that Cisd2 regulates intracellular Ca2+ homeostasis and the redox status of various types of cell. Citation3 , Citation4 Intriguingly, our recent study has revealed that Cisd2, which is located within the most frequently deleted region of chromosome 4q in HCC patients, is a novel haploinsufficient tumor suppressor gene. Citation5 Loss of only a single allele of Cisd2 in mice, which mimics the hemizygous status of CISD2 in HCC patients, results in cells that are functionally unable to maintain normal hepatocyte metabolism and this leads to NAFLD/NASH in mice. Cisd2+/- mice also develop a low incidence of spontaneous HCC as well as accelerate HCC mediated by either hepatitis B virus X protein (HBx) or induced by diethylnitrosamine (DEN); conversely, the presence of a Cisd2 transgene significantly delays the onset of either HBx-mediated or DEN-induced hepatocarcinogenesis. This suggests that Cisd2 acts as a safeguard and protects against tumor emergence. Mechanistically, Cisd2 interacts with Serca2b and modulates its redox status helping maintain optimal Serca2b activity in hepatocytes. Thus Cisd2 haploinsufficiency will impair Serca2b activity and disrupt Ca2+ homeostasis, which leads to NAFLD and promotes HCC development (). In HCC patients, loss of heterozygosity, as well as down-regulation of CISD2, have been frequently observed in HCC tissue compared with adjacent non-tumor tissue. Taken together, these findings reveal that Cisd2 haploinsufficiency is a factor that promotes hepatocarcinogenesis; this pinpoints Cisd2 as a haploinsufficient tumor suppressor in HCC. Our findings form the basis of a new paradigm for the function of Cisd2 in the liver and the etiology of HCC and suggest that they can be used to develop therapeutic strategies for the treatment of NAFLD/NASH, thus preventing malignant progression to HCC.
Figure 1. CISD2 as a potential therapeutic drug target for the treatment of NAFLD and NASH, and the prevention of HCC. A, In mice, CDGSH iron sulfur domain 2 (Cisd2) haploinsufficiency impairs sarcoplasmic/endoplasmic reticulum Ca2+ ATPase 2 isoform b (Serca2b) activity and disrupts Ca2+ homeostasis leading to non-alcoholic fatty liver disease (NAFLD). While obesity impairs Serca2b activity via a decrease in Serca2b protein level. B, In human, CISD2 and SERCA2b are both possible drug targets for the treatment of NAFLD and the prevention of hepatocellular carcinoma (HCC). In addition to directly target SERCA2b, CISD2 activators may have the potential to treat NAFLD indirectly by enhancing SERCA2b activity through an increase in CISD2 protein level. ER, endoplasmic reticulum; HBV, hepatitis B virus; HFD, high fat diet; NASH, nonalcoholic steatohepatitis.
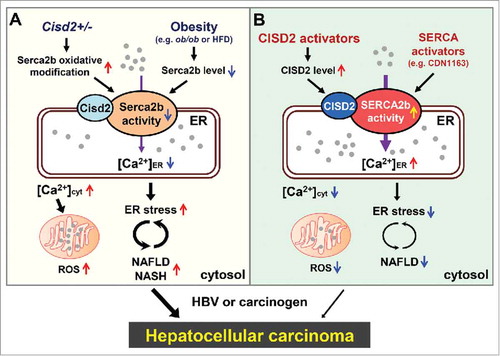
Activation of CISD2 as a promising therapeutic strategy for treating NAFLD/NASH and preventing HCC. NAFLD/NASH is the most common chronic liver disease and is one of the main risk factors for HCC. However, despite the high prevalence of this disease and the high risk of serious progression regarding clinic outcome, namely fibrosis and HCC, currently there is no therapeutic agent approved for the efficient treatment of NAFLD. Finding therapeutic compounds that are able to effectively increase Cisd2 expression might have potential as a therapeutic strategy for the treatment of NAFLD. Using an ob/ob obesity-related NAFLD model, NAFLD can be improved by treatment with the SERCA activator CDN1163, which suggests that activation of SERCA2b by a drug can be a target when developing treatments for NAFLD. Citation6 Since Cisd2 is a positive modulator for Serca2b enzymatic activity, we propose that an increased CISD2 level, when induced by a CISD2 activator (for example, small-molecule compound), should enhance the activity of SERCA2b, thus restoring ER Ca2+ homeostasis and reversing NAFLD ().
Targeting ER stress and oxidative stress using natural compounds and/or synthetic molecules had been shown to be a good approach for NAFLD therapy. Citation7 , Citation8 Interestingly, the naturally derived antioxidant curcumin, which is known to have a beneficial effect by improving liver metabolism and ameliorating NAFLD in the rodent models and human patients, is a Cisd2 activator. Citation9 Accordingly, it is of great interest to identify if other antioxidants and ER stress inhibitors have a beneficial effect on NAFLD functioning in a Cisd2-dependent manner or if there is interplay between the mechanism of action of these compounds and Cisd2 in the liver.
Disclosure of potential conflicts of interest
No potential conflicts of interest are disclosed.
Acknowledgments
We acknowledge support from the Ministry of Science and Technology (NSC99-2628-B-010-001-MY3, MOST 103-2633-B-400-002, and MOST 104-3011-B-010-001) and a grant from the Ministry of Education, Aim for the Top University Plan.
Additional information
Funding
References
- Ma C , Zhang Q , Greten TF. Nonalcoholic fatty liver disease promotes hepatocellular carcinoma through direct and indirect effects on Shepatocytes. FEBS J. 2018;285(4):752–62. doi:10.1111/febs.14209. PMID:28857485.
- Chemaly ER , Troncone L , Lebeche D . SERCA control of cell death and survival. Cell Calcium. 2018;69:46–61. doi:10.1016/j.ceca.2017.07.001. PMID:28747251
- Wang CH , Kao CH , Chen YF , Wei YH , Tsai TF . Cisd2 mediates lifespan: is there an interconnection among Ca2+ homeostasis, autophagy, and lifespan? Free Radic Res. 2014;48(9):1109–14. doi:10.3109/10715762.2014.936431. PMID:24974737
- Huang YL , Shen ZQ , Wu CY , Teng YC , Liao CC , Kao CH , Chen LK , Lin CH , Tsai TF . Comparative proteomic profiling reveals a role for Cisd2 in skeletal muscle aging. Aging Cell. 2017;e12705. https://doi.org/10.1111/acel.12705. PMID:29168286.
- Shen ZQ , Chen YF , Chen JR , Jou YS , Wu PC , Kao CH , Wang CH , Huang YL , Chen CF , Huang TS , et al. CISD2 haploinsufficiency disrupts calcium homeostasis, causes nonalcoholic fatty liver disease, and promotes hepatocellular carcinoma. Cell Rep. 2017;21(8):2198–211. doi:10.1016/j.celrep.2017.10.099. PMID:29166610.
- Kang S , Dahl R , Hsieh W , Shin A , Zsebo KM , Buettner C , Hajjar RJ , Lebeche D . Small molecular allosteric activator of the sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) attenuates diabetes and metabolic disorders. J Biol Chem. 2016;291(10):5185–98. doi:10.1074/jbc.M115.705012. PMID:26702054.
- Wu FL , Liu WY , Van Poucke S, Braddock M , Jin WM , Xiao J , Li XK , Zheng MH . Targeting endoplasmic reticulum stress in liver disease. Expert Rev Gastroenterol Hepatol. 2016;10(9):1041–52. doi:10.1080/17474124.2016.1179575. PMID:27093595
- Salomone F , Godos J , Zelber‐Sagi S . Natural antioxidants for non‐alcoholic fatty liver disease: molecular targets and clinical perspectives. Liver Int. 2016;36(1):5–20. doi:10.1111/liv.12975. PMID:26436447
- Lin CC , Chiang TH , Chen WJ , Sun YY , Lee YH , Lin MS . CISD2 serves a novel role as a suppressor of nitric oxide signalling and curcumin increases CISD2 expression in spinal cord injuries. Injury. 2015;46(12):2341–50. doi:10.1016/j.injury.2015.07.040. PMID:26387034
