Abstract
ER stress-mediated induction of a new vertebrate-specific autophagy cargo receptor, CCPG1 (cell-cycle progression gene 1), drives degradation of endoplasmic reticulum. CCPG1 acts via ATG8-family interaction and, non-canonically, via discrete interactions with FIP200. CCPG1 ameliorates ER stress in the exocrine pancreas. This has potential implications for inflammation and cancer, discussed here.
Main text
Selective macroautophagy (hereafter autophagy) is a cellular homeostatic mechanism that acts to degrade damaged organelles from the cytosol. A key mechanistic feature is the selection of cytosolic cargoes for preferential sequestration into nascent double-membraned transport vesicles called autophagosomes. In mammals, this occurs via cargo receptors, proteins that bind autophagy membrane-associated proteins, typically the ATG8 (autophagy-related 8)-family of ubiquitin-like proteins (a collective term in mammals for proteins of the LC3 and GABARAP subfamilies) (Khaminets, Behl et al. Citation2016). Receptors also bind directly or indirectly to cargoes, thus recruiting them into the forming vesicle. Autophagosomes fuse with lysosomes, resulting in hydrolytic degradation of cargoes.
Loss of autophagy is toxic to, or potentially sensitises to stress, a variety of adult mammalian tissue types (Yoshii, Kuma et al. Citation2016). Notably, a subset of such tissues contains those with high biosynthetic rates coupled to large secretory enzyme flux through the endoplasmic reticulum. For example, pancreatic acinar cells, the digestive enzyme-secreting cells of the exocrine pancreas, produce large amounts of inactive enzyme in the form of zymogens. These pro-proteins are packaged into dense core vesicles after transit through the ER (endoplasmic reticulum) and Golgi. Cell death of acinar cells can potentially lead to tissue damage and inflammation, via aberrant release of these hydrolytic enzymes. These cell types are sensitive to ER stress, which can be defined here as the hyper-accumulation of unfolded or aggregated protein within the ER lumen, leading to engagement of homeostatic signalling pathway(s) (Smith and Wilkinson Citation2017). The most well understood such pathway is the UPR (unfolded protein response), which acts to halt general translation and upregulate the transcription of genes that resolve ER stress. The UPR is basally engaged in pancreatic acinar cells at a level compatible with ongoing cell function. High-levels of UPR signalling can drive apoptosis if ER stress is not resolved.
Mouse models where autophagy is ablated in the exocrine pancreas exhibit rapid degeneration of the acinar cell compartment and concomitant inflammation, and sensitivity to oncogene-driven formation of regions of acinar-ductal metaplasia (ADM) and pancreatic intraepithelial neoplasia (PanINs) (Rosenfeldt, O'Prey et al. Citation2013, Yang, Rajeshkumar et al. Citation2014, Diakopoulos, Lesina et al. Citation2015). ADM and PanINs are putative precursor lesions of pancreatic ductal adenocarcinoma (PDAC) that derive from acinar cells. At the cellular level, loss of autophagy function in pancreatic acinar cells is associated with multiple pathologies (Antonucci, Fagman et al. Citation2015). Cells accumulate insoluble protein aggregates and defective mitochondria. It is likely that these impair cellular bioenergetics and thus contribute to cell death and inflammation. However, the ER of such cells also becomes expanded and distended, and molecular markers indicative of hyper-engagement of the UPR are detectable. However, it is not clear whether this is due to a defect in ER-phagy or to the aforementioned collapse in cellular energetics.
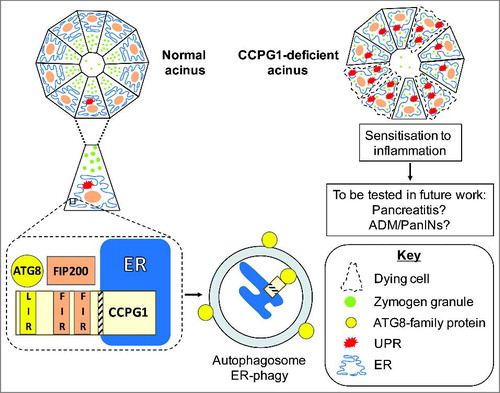
We recently described a new cargo receptor that mediates ER-phagy, CCPG1 (cell cycle progression gene 1) (Smith, Harley et al. 2017). CCPG1 transcripts are elevated upon experimental induction of ER stress in human cells in vitro, indicating a role in ER homeostatic mechanisms. Indeed, under ER stress conditions, CCPG1 was demonstrated to be required for ER turnover in an ATG5 (autophagy-related 5)-dependent manner. ATG5 is a core autophagy protein, which is required for all forms of autophagy within cells. CCPG1 executes this function via what we termed a “non-canonical” cargo receptor function (). In detail, CCPG1 is a transmembrane protein embedded in the ER and exposes a LIR (LC3-interacting region) peptide motif to the cytosol for interaction with ATG8-family proteins, as conventional mammalian cargo receptors do. However, it also binds directly to the protein FIP200 (FAK-interacting protein 200 kDa), another key component of the autophagy pathway that is recruited to early autophagic intermediates. Interestingly, autophagy is an evolutionarily conserved process and, although no orthologue of FIP200 is detectable in yeast, the region CCPG1 binds to within FIP200 has homology to the yeast protein Atg11. In yeast, Atg11 and Atg8 interactions are important in cargo selection in a multi-step process. Atg11 may recruit cargo to machinery that drives localised accumulation of Atg8 (Torggler, Papinski et al. Citation2016). We showed independent roles for both the ATG8-family and FIP200 interactions of CCPG1 in recruitment into autophagosomes and for stimulation of ER-phagy, posing the question of whether CCPG1 acts in an analogous manner to yeast selective autophagy receptors. This must be further explored.
In vivo, CCPG1 loss leads to distension and disrupted cellular distribution of the ER within the pancreatic acinar cells of mice (). Elevation of molecular markers of ER stress also occurs, consistent with a loss of ER-phagic regulation of ER homeostasis. However, it is possible that CCPG1 combines a role in ER-phagy with other, undiscovered functions in ER homeostasis. This should be a topic for future study. Importantly, in older CCPG1-deficient mice, evidence of sensitivity to inflammation was obtained. Elevated basal rates of cell death and compensatory proliferation of healthy acinar cells correlated with the appearance, albeit rare, of sporadic infiltrates of inflammatory cells. Thusly, it is possible that CCPG1 loss would also sensitise to overt inflammatory insults, which include molecular lesions such as KRAS (Kirsten-Ras) proto-oncogene mutation and activation. KRAS oncoprotein co-operates with additional inflammatory stimuli to drive both ADM and PanIN formation (Daniluk, Liu et al. Citation2012).
Finally, future discovery of other partners of CCPG1, such as protein-protein interactors or functional genetic interactors acting in ER homeostasis, may shine light on a network of proteins involved in regulating pancreatic exocrine cell health and disease. CCPG1 itself, or interacting partners, may ultimately prove good therapeutic targets for manipulating ER homeostasis and consequently inflammation or cancer development.
Cells within pancreatic acini maintain a healthy ER via CCPG1 (cell-cycle progression gene 1), which resides within the membrane of this organelle, presenting discrete ATG8-family and FIP200 interacting peptide motifs to the cytosol. These motifs are termed the LIR (LC3-interacting region) and FIRs (FIP200-interacting regions), respectively. The LIR and FIRs facilitate ER-phagy, the selective sequestration of ER material into autophagosomes. ER homeostasis is aberrant in the absence of CCPG1 protein in these cells, resulting in accumulated and distended ER, and engagement of the UPR (unfolded protein response). Consistent with such stress, acinar cells are prone to cell death and sporadic inflammatory infiltrates are detected within aged, CCPG1-deficient pancreata. This is presumably reflective of the loss of ER-phagy capacity of pancreatic acinar cells, although it is possible that CCPG1 has yet further functions in ER homeostasis. An outstanding question is whether the control of ER homeostasis, and thus sensitivity to cell death and inflammation, will impact upon pre-disposition to inflammation-related disease states, including acute or chronic pancreatitis, and pre-neoplastic and neoplastic lesions (ADM = acinar-ductal metaplasia, PanIN = pancreatic intraepithelial neoplasia).
Orcid
Simon Wilkinson: 0000-0003-1082-8218
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Antonucci, L., J. B. Fagman, J. Y. Kim, J. Todoric, I. Gukovsky, M. Mackey, M. H. Ellisman and M. Karin. Basal autophagy maintains pancreatic acinar cell homeostasis and protein synthesis and prevents ER stress. Proc Natl Acad Sci U S A. 2015;112:E6166–6174.
- Daniluk, J., Y. Liu, D. F. Deng, J. Chu, H. J. Huang, S. Gaiser, Z. Cruz-Monserrate, H. M. Wang, B. A. Ji and C. D. Logsdon. An NF-kappa B pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–1528.
- Diakopoulos, K. N., M. Lesina, S. Wormann, L. Song, M. Aichler, L. Schild, A. Artati, W. Romisch-Margl, T. Wartmann, R. Fischer et al. Impaired autophagy induces chronic atrophic pancreatitis in mice via sex- and nutrition-dependent processes. Gastroenterology. 2015;148:626–638 e617.
- Khaminets, A., C. Behl and I. Dikic. Ubiquitin-dependent and independent signals in selective autophagy. Trends Cell Biol. 2016;26:6–16.
- Rosenfeldt, M. T., J. O'Prey, J. P. Morton, C. Nixon, G. MacKay, A. Mrowinska, A. Au, T. S. Rai, L. Zheng, R. Ridgway, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300.
- Smith, M. and S. Wilkinson. ER homeostasis and autophagy. Essays Biochem. 2017;61:625–635.
- Smith, M. D., M. E. Harley, A. J. Kemp, J. Wills, M. Lee, M. Arends, A. von Kriegsheim, C. Behrends and S. Wilkinson. CCPG1 Is a non-canonical autophagy cargo receptor essential for ER-phagy and pancreatic ER proteostasis. Dev Cell. 2018;44:1–16
- Torggler, R., D. Papinski, T. Brach, L. Bas, M. Schuschnig, T. Pfaffenwimmer, S. Rohringer, T. Matzhold, D. Schweida, A. Brezovich et al. Two independent pathways within selective autophagy converge to activate atg1 kinase at the vacuole. Mol Cell. 2016;64:221–235.
- Yang, A., N. V. Rajeshkumar, X. Wang, S. Yabuuchi, B. M. Alexander, G. C. Chu, D. D. Von Hoff, A. Maitra and A. C. Kimmelman. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4:905–913.
- Yoshii, S. R., A. Kuma, T. Akashi, T. Hara, A. Yamamoto, Y. Kurikawa, E. Itakura, S. Tsukamoto, H. Shitara, Y. Eishi et al. Systemic analysis of Atg5-null mice rescued from neonatal lethality by transgenic ATG5 expression in Neurons. Dev Cell. 2016;39:116–130.