ABSTRACT
Although downregulation of DICER – a critical enzyme in microRNA (miRNA) maturation – reportedly promotes cancer metastasis, understanding of its upstream regulators remains limited. Our recent study demonstrated a noncanonical oncogenic effect of hypoxia-inducible factor-1α (HIF-1α), which directly binds with DICER to promote PARKIN-mediated autophagic–lysosomal proteolysis and consequently suppresses miRNA biogenesis, facilitating metastasis.
Keywords:
MicroRNAs (miRNAs) are endogenous short single-stranded RNAs that post-transcriptionally suppress target gene expression to regulate cellular functions. Dysregulation of miRNAs has been implicated in human malignancies.Citation1 DICER is a central enzyme that processes miRNA precursors into mature miRNAs. Deletion of Dicer1 in a mouse model enhanced tumorigenesis.Citation2 Supporting to this concept, downregulation of DICER expression was observed in human cancers and has been identified to promote cancer metastasis and tumorigenesis due to repression of global miRNA maturation,Citation3,Citation4 but the upstream mechanisms are not well understood.
We first identified DICER as a novel hypoxia-inducible factor-1α (HIF-1α)-binding protein.Citation5 HIF-1α, as its name implies, is a critical oxygen-sensitive transcription factor induced and stabilized under hypoxic conditions. Tissue hypoxia is a microenvironmental feature under inadequate oxygen supply in a region of the body. As a tumor rapidly grows, the vascular supply to the massive number of cancer cells becomes too low, primarily in regions with insufficient oxygen. Activation of HIF-1α contributes to epithelial–mesenchymal transition (EMT), enabling the escape of cells from the hypoxic region.Citation6 HIF-1α promotes malignant phenotypes of cancer cells that depend on the transactivation of downstream oncogenic transcripts. Our findings showed that growth factor- and hypoxia-induced HIF-1α post-translationally downregulate DICER protein stability, regardless of its transactivation ability. This regulation indicates a transcription-independent function of HIF-1α in response to biological stimulations. Furthermore, we demonstrated that HIF-1α-mediated DICER downregulation occurs through the autophagic–lysosomal pathway rather than through proteosomal degradation. HIF-1α recruits E3 ligase PARKIN to ubiquitinate DICER, which is selectively recognized by autophagosome receptor P62. Moreover, HIF-1α-mediated Dicer downregulation inhibits miR-200b maturation, subsequently enhances ZEB1-mediated EMT and metastasis of colon cancer in a mouse model.
Our findings identify a noncanonical oncogenic role of HIF-1α in suppressing miRNA biogenesis by facilitating the binding of PARKIN and DICER, whereby ubiquitinated DICER is selectively degraded through autophagy ().Citation5 Recent study revealed that PARKIN is a novel E3 ligase for ubiquitination of HIF-1α. Ubiquitinated HIF-1α is further degraded by proteasome to inhibit cancer metastasis, suggesting that PARKIN serves as a tumor suppressor during cancer progression.Citation7 Given our observation that HIF-1α is strongly induced by biological stimulations, HIF-1α mediates the interaction of PARKIN and DICER and activates an oncogenic effect that enhances DICER ubiquitination to suppress miRNA biogenesis for cancer metastasis. Under such a scenario, HIF-1α may recruit DICER as a scapegoat for PARKIN-mediated ubiquitination to escape degradation and concomitantly inhibit the tumor-suppressive function of DICER. By contrast, PARKIN-mediated ubiquitination of endogenous HIF-1α is sufficient for its proteasomal degradation, and DICER expression is mutually maintained. These regulations indicate that HIF-1α functions as a signaling hub that coordinates miRNA maturation in response to microenvironmental stimulation.
Figure 1. Two synergistic modes for DICER downregulation. HIF-1α (hypoxia-inducible factor-1α)-independent mechanism: Under hypoxia, the function of oxygen-sensitive demethylases KDM6A/B is inactivated and methyltransferase EZH2 activity increases for H3K27me3, suppressing transcription of DICER1.Citation7 HIF-1α-dependent mechanism: Hypoxia-, IGF (Insulin-like growth factor)- and EGF (Epidermal growth factor)-induced HIF-1α mediate and enhance the binding of DICER and PARKIN. The interaction ubiquitinates DICER protein for autophagic–lysosomal degradation. These modes work synergistically to inhibit DICER expression, suppressing miRNA maturation. Subsequently, downregulation of miR-200b promotes ZEB1-mediated EMT of cells and cancer metastasis.Citation5
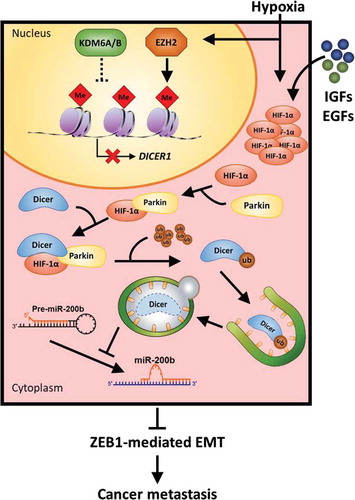
Apart from our results, transcriptional inhibition of DICER1 under hypoxia has been observed in several studies.Citation5,Citation8,Citation9,Citation10 We discovered that DICER protein stability is decreased in HIF-1α-overexpressing cells under both hypoxic and nonhypoxic stimulation. However, alternation of DICER1 mRNA levels was only observed under hypoxic conditions, suggesting the existence of another regulatory pathway leading to suppressing transcriptional levels of DICER1. Van den Beucken et al. Citation9 demonstrated an HIF-1α-independent mechanism in downregulation of DICER under hypoxic conditions. They revealed that basal levels of DICER are regulated by the opposing activity of the oxygen-sensitive H3K27me3 demethylases KDM6A, KDM6B, and methyltransferase EZH2. The activity of KDM6A/B is decreased and EZH2 is upregulated under hypoxia, resulting in enhanced H3K27me3 silencing the DICER1 promoter in an HIF-1α-independent manner. The study uncovered an HIF-1α-independent mechanism for enhancement of stem cell phenotypes through epigenetic regulation of DICER1 under hypoxic conditions.Citation9 In response to tumor hypoxia, DICER is suppressed in HIF-1α-independent epigenetic regulation through the enhancement of H3K27me3 at the DICER1 promoterCitation9 and concurrently inhibited through post-translational regulation in an HIF-1α-dependent mechanism that through which HIF-1α interacts with DICER to promote PARKIN-mediated autophagic–lysosomal proteolysis.Citation5 By contrast to hypoxia, growth factor stimulation also induces HIF-1α-mediated DICER proteolysis without mediating epigenetic repression of DICER1 (). Both regulations cooperatively facilitate ZEB1-mediated EMT for cancer metastasis through suppression of miR-200b maturation. This evidence not only demonstrates dual upstream regulatory modes of DICER suppression but also provides new insight for understanding the mechanism of miRNA dysregulation in cancer.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
References
- Lin S, Gregory RI. MicroRNA biogenesis pathways in cancer. Nat Rev Cancer. 2015; (15):321–333. PMID:25998712. doi:10.1038/nrc3932.
- Kumar MS, Pester RE, Chen CY, Lane K, Chin C, Lu J, Kirsch DG, Golub TR, Jacks T. Dicer1 functions as a haploinsufficient tumor suppressor. Genes Dev. 2009; (23):2700–2704. PMID:19903759. doi:10.1101/gad.1848209.
- Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nat Genet. 2007; (39):673–677. PMID:17401365. doi:10.1038/ng2003.
- Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010; (141):1195–1207. PMID:20603000. doi:10.1016/j.cell.2010.05.017.
- Lai HH, Li JN, Wang MY, Huang HY, Croce CM, Sun HL, Lyu YJ, Kang JW, Chiu CF, Hung MC, et al. HIF-1α promotes autophagic proteolysis of Dicer and enhances tumor metastasis. J Clin Invest. 2018; (128):625–643. PMID:29251629. doi:10.1172/JCI89212.
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003; (3):721–732. PMID:13130303. doi:10.1038/nrc1187.
- Liu J, Zhang C, Zhao Y, Yue X, Wu H, Huang S, Chen J, Tomsky K, Xie H, Khella CA, et al. Parkin targets HIF-1alpha for ubiquitination and degradation to inhibit breast tumor progression. Nat Commun. 2017; (8):1823. PMID:29180628. doi:10.1038/s41467-017-01947-w.
- Rupaimoole R, Wu SY, Pradeep S, Ivan C, Pecot CV, Gharpure KM, Nagaraja AS, Armaiz-Pena GN, McGuire M, Zand B, et al. Hypoxia-mediated downregulation of miRNA biogenesis promotes tumour progression. Nat Commun. 2014; (5):5202. PMID:25351346. doi:10.1038/ncomms6202.
- van den Beucken T, Koch E, Chu K, Rupaimoole R, Prickaerts P, Adriaens M, Voncken JW, Harris AL, Buffa FM, Haider S, et al. Hypoxia promotes stem cell phenotypes and poor prognosis through epigenetic regulation of DICER. Nat Commun. 2014; (5): 5203. PMID:25351418. doi:10.1038/ncomms6203.
- Ho JJ, Metcalf JL, Yan MS, Turgeon PJ, Wang JJ, Chalsev M, Petruzziello-Pellegrini TN, Tsui AK, He JZ, Dhamko H, et al. Functional importance of Dicer protein in the adaptive cellular response to hypoxia. J Biol Chem. 2012; (287):29003–29020. PMID:22745131. doi:10.1074/jbc.M112.373365.
