ABSTRACT
Response to targeted therapies is limited by the activation or inhibition of feedback loops. Here we report the ubiquitin specific peptidase 28/F-box WD repeat-containing protein 7 (USP28/FBW7) complex functions as a negative regulator of mitogen-activated protein kinase (MAPK) pathway by targeting v-raf murine sarcoma viral oncogene homolog B (BRAF) for degradation, a process which is lost in a large proportion of BRAF mutant melanoma patients, resulting in resistance to BRAF inhibitor therapies.
Commentary
The generation of inhibitors selectively targeting v-raf murine sarcoma viral oncogene homolog B (BRAF) V600E mutations has revolutionized the treatment of melanoma patients harbouring this lesion with the majority of patients exhibiting marked and prolonged responses. Unfortunately, however, rarely do tumours completely regress limiting the overall response rates of these compounds. One of the major reasons for this is that targeted therapy with kinase inhibitors induces complex compensatory mechanisms, also known as adaptive responses or feedback loops, permitting a small proportion of the original tumour cell population to survive and eventually to proliferate.
Mitogen-activated protein kinase (MAPK) signalling pathway controls various cellular functions including proliferation, transformation, differentiation and migration. To accurately reflect cues from the extracellular environment, MAPK mediated transcriptional responses are tightly regulated by a number of different compensatory mechanisms. One example of which involves the dual specific protein phosphatase (DUSP). DUSP is transcriptionally induced following MAPK activation whereby it functions through a negative feedback loop to dephosphorylate and inactive ERK1 and ERK2, resulting in downregulation of the pathway.Citation1 Interestingly, it has been noted that tumour responses in BRAF V600E melanoma patients to BRAF inhibitors are directly correlated with pERK downregulation.Citation2 It is therefore unsurprising that activating mutations or upregulation of components of MAPK pathway have been frequently reported as mechanisms of resistance to BRAF inhibitors in the clinic.Citation3
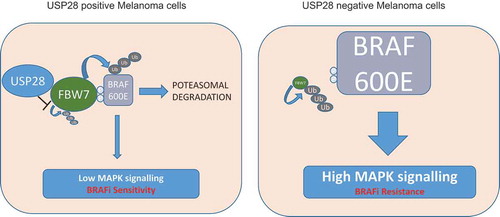
Adaptive responses regulating deubiquitinating enzyme (DUBs) activity has been shown to drive signalling in a number of oncogenic pathways.Citation4 In this regard we reasoned that BRAF inhibitor sensitivity in melanoma may be regulated by DUBs. Our recent results show that ubiquitin specific peptidase 28 (USP28) expression is enhanced following treatment with vemurafenib, a clinically approved BRAF(V600E) inhibitor, whereby USP28 acts in conjunction with the F-box WD repeat containing protein 7 (FBW7) complex to target BRAF for degradation.Citation5 In contrast, loss of USP28 expression led to BRAF stabilisation and downstream activation of canonical MAPK pathway (). FBW7 is a component of SKP1/CUL1/F-box (SCF) E3 ligase complex and acts as a substrate recognition subunit binding to phosphorylated degron sites of various oncogenes such as MYC, NOTCH1, JUN, Cyclin E.Citation6 The interaction of FBW7 with its substrates induces SCF mediated ubiquitination of bound proteins leading to their proteasomal mediated degradation. However, FBW7 itself is targeted by the SCF complex resulting in its own degradation. USP28 interacts with and deubiquitinates FBW7 leading to the stability of FBW7 and consequently promoting degradation of FBW7 substrates. On the other hand, in circumstances where USP28 levels are high, USP28 can deubiquitinate and stabilize both FBW7 and its bound substrate.Citation7 Thus, supporting a role for USP28 as both a tumour suppressor and oncogene with our own study validating a tumour suppressor role of USP28 in melanoma.Citation8
Figure 1. Vemurafenib resistance in melanoma cells lacking USP28. Under normal conditions ubiquitin specific peptidase 28 (USP28) functions to stabilise F-box WD repeat-containing protein 7 (FBW7) resulting in SKP1/CUL1/F-box (SCF) mediated downregulation of targeted substrates including v-raf murine sarcoma viral oncogene homolog B (BRAF, left panel). Under USP28 depleted conditions BRAF stabilisation enhances mitogen-activated protein kinas (MAPK) pathway activation and resistance to BRAF inhibitors (right panel).
Recently, inactivating mutations in FBW7 have been identified in melanoma correlating with poor prognosis.Citation9 Importantly, our own analysis indicates that USP28 expression is downregulated in a large proportion of melanoma patients with the majority of these patients harbouring mutations in BRAF (V600E), neurofibromatosis type 1 (NF1), neuroblastoma RAS viral oncogene homolog (NRAS), supporting a role for USP28 loss in melanoma progression. Furthermore, in vitro and in vivo studies of BRAF mutant melanoma cell lines showed that USP28 depletion enhanced phosphorylated ERK levels leading to decreased vemurafenib sensitivity. Furthermore, diminished USP28 levels inhibited vemurafenib induced apoptosis through the downregulation of pro-apoptotic proteins including BIM. These observations were further supported by the observation of increased time to progression in melanoma patients with high levels of USP28 in response to BRAF/MEK inhibition therapy suggesting a role for USP28 as a potential biomarker for vemurafenib resistance.
In a search to characterize synthetic lethal interactions in melanoma cell lines depleted for USP28 we identified the polo-like kinase 1 (PLK1) inhibitor, rigosertib. Treatment with rigosertib specifically enhanced apoptosis in USP28 depleted cell lines compared to wild type cell lines. Interestingly, rigosertib like drugs such as nocodazole, which are able to induce cell cycle arrest, were projected out in our drug screening analysis as synthetic lethal hits with the loss of USP28 enzyme. Although, recent studies have shown that these anticancer drugs are able to trigger c-Jun N-terminal kinase (JNK) mediated phosphoregulatory circuits to inhibit RAS dependent activation of MAPK signalling,Citation10 future studies are required to identify the specific mechanism of action of rigosertib in targeting USP28 deleted melanoma cells in a RAS independent activation context in which BRAF(V600E) mutant kinase acts as a monomer to promote the tumorigenic behaviour of cancer cells in melanoma.
At present a number of USP28 inhibitors are under development. As USP28 is differently expressed in different tissues, downregulation of USP28 under certain conditions may be a promising therapeutic strategy to downregulate FBW7 substrates. For example, in intestinal cancers, where USP28 is highly expressed, the FBW7 substrate MYC proto-oncogene (c-MYC) may be a direct target for USP28 deubiquitination activity leading to c-MYC stabilisation in these tissues. Effective targeting of USP28 may lead to c-MYC downregulation and a reversal of c-MYC mediated transformation. However, in tissues such as skin which exhibit low expression of USP28, the enzymatic activity of USP28 is confined to FBW7 leading to the degradation of oncogenes such as BRAF. Deciphering the molecular context of USP28 in different tissues will be of great importance to maximizing the clinical efficacy of USP28 inhibitors. Furthermore, our findings also point to the use of rigosertib as a potential therapeutic strategy to target USP28 depleted melanoma cells.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Additional information
Funding
References
- Caunt CJ, Keyse SM. Dual-specificity MAP kinase phosphatases (MKPs): shaping the outcome of MAP kinase signalling. FEBS J. 2013;280(2):489–504. doi:10.1111/j.1742-4658.2012.08716.x.
- Bollag G, Hirth P, Tsai J, Zhang J, Ibrahim PN, Cho H, Spevak W, Zhang C, Zhang Y, Habets G, et al., Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467(7315):596–599. doi:10.1038/nature09454.
- Lito P, Rosen N, Solit DB. Tumor adaptation and resistance to RAF inhibitors. Nat Med. 2013;19(11):1401–1409. doi:10.1038/nm.3392.
- Kumari N, Jaynes PW, Saei A, Iyengar PV, Richard JLC, Eichhorn PJA. The roles of ubiquitin modifying enzymes in neoplastic disease. Biochim Biophys Acta. 2017.
- Saei A, Palafox M, Benoukraf T, Kumari N, Jaynes PW, Iyengar PV, Muñoz-Couselo E, Nuciforo P, Cortés J, Nötzel C, et al. Loss of USP28-mediated BRAF degradation drives resistance to RAF cancer therapies. J Exp Med. 2018. doi:10.1084/jem.20171960.
- Welcker M, Clurman BE. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nature Reviews Cancer. 2008;8(2):83. doi:10.1038/nrc2290.
- Schülein-Völk C, Wolf E, Zhu J, Xu W, Taranets L, Hellmann A, Jänicke LA, Diefenbacher ME, Behrens A, Eilers M, et al., Dual regulation of Fbw7 function and oncogenic transformation by Usp28. Cell Rep. 2014;9(3):1099–1109. doi:10.1016/j.celrep.2014.09.057.
- Diefenbacher ME, Popov N, Blake SM, Schülein-Völk C, Nye E, Spencer-Dene B, Jaenicke LA, Eilers M, Behrens A. The deubiquitinase USP28 controls intestinal homeostasis and promotes colorectal cancer. J Clin Invest. 2014;124(8):3407–3418. doi:10.1172/JCI73733.
- Aydin IT, Melamed RD, Adams SJ, Castillo-Martin M, Demir A, Bryk D, Brunner G, Cordon-Cardo C, Osman I, Rabadan R, et al., FBXW7 mutations in melanoma and a new therapeutic paradigm. J Natl Cancer Inst. 2014;106(6):dju107. doi:10.1093/jnci/dju061.
- Ritt DA, Abreu-Blanco MT, Bindu L, Durrant DE, Zhou M, Specht SI, Stephen AG, Holderfield M, Morrison DK., Inhibition of Ras/Raf/MEK/ERK pathway signaling by a stress-induced phospho-regulatory circuit. Molecular Cell. 2016;64(5):875–887. doi:10.1016/j.molcel.2016.10.029.
