ABSTRACT
The structural basis of blocking human epidermal growth factor receptor-2 (HER2) dimerization remains of great interest to generate effective anti-cancer therapies. Despite clinically feasible outcome in mammary tumors, a fine consensus between efficacy and safety remains a critical challenge beyond breast cancer. Here we extrapolate on the balancing act using recently reported clinical findings in salivary ductal carcinomas.
KEYWORDS:
Salivary ductal carcinomas (SDCs) are rare but highly aggressive salivary gland cancers with limited treatment options of surgery and radiotherapy. Between 30% and 40% of SDC patients have been found positive for human epidermal growth factor receptor-2 (HER2).Citation1 In addition, ~32% of SDC patients have gain of function mutations or alternations in the HER2 gene, making it an attractive target for immunotherapy.Citation1
Since last two decades, HER2 has been of great significance for various epithelial cancers that include breast, ovarian pancreatic and colon. Similar to other members of epidermal growth factor receptor (EGFR) family, HER2 activation is highly dependent upon its dimerization and autophosphorylation and United States Federal Drug Administration (USFDA) has approved various HER2 specific monoclonal antibodies (such as trastuzumab and pertuzumab) as a single agent or in combination of chemotherapy against breast cancers. In addition, trastuzumab emtansine, an antibody-drug conjugate (ADC) is highly effective against late-stage aggressive breast cancers. Primarily, various investigational clinical anti-HER2 antibodies selectively block HER2 dimerization and downstream oncogenic signaling in breast and other cancers of epithelial origin.
A recent phase-II single-arm and single-center clinical trial study positively reported simultaneous dosing of trastuzumab (humanized anti-HER2 antibody) and docetaxel (a chemotherapy drug) as an effective treatment strategy for HER2+ SDC patients.Citation1 Out of registered 57 patients (HER2 expression being the major inclusion criteria), 40 patients showed either partial or complete remission, 14 of them had stable disease, while only three showed progressive cancer.Citation1 Given the extremely poor prognosis of SDCs, a highly encouraging median progression-free survival (PFS) of 8.9 months (90% confidence interval, CI) was reported.
Despite the small clinical trial size (sample) and study being non-randomized, the described results of combinatorial systemic therapies are highly promising for thousands HER2+ SDC worldwide lacking effective and long-term treatment options worldwide. However, in terms of safety, 91% treated patients were presented with anemia and ~88% patients experienced significantly decreased white blood cells (WBC) count.Citation1 Although no death occurred due to the treatment, the reported grade-4 level toxicities (neutropenia in particular) were observed in ~60% of the patients. Strikingly, the reported adverse effects and toxicities in SDC trialCitation1 were worse in Asian population than similar trials of trastuzumab and docetaxel in EuropeCitation2 or United StatesCitation3 against breast cancer patients.
In addition to primary downregulation of oncogenic HER2 signaling, the secondary anti-tumor function of trastuzumab has been partially attributed to antibody-dependent cellular cytotoxicity (ADCC) and antibody-dependent cellular phagocytosis (ADCP) of tumor cells.Citation4 Both ADCC and ADCP require selective engagement of antibody fragment crystallizable region (Fc) binding to selective receptors called cluster of differentiation 16 (CD16, also known as FcRγIII) on immune effector cells. Considering the differential and reduced toxicity in Caucasian population as compared to Asian population,Citation2,Citation3 and published reports of FcR receptor polymorphism being the major susceptibility gene for lupus and other immunological disorders in the Asian population,Citation5 would differential engineering of clinical antibodies with variable affinity against FcR’s expressed in Asian population (as compared to Caucasian) has potential to limit the described adverse effects. Specifically, could differentially engagement of CD16 receptor isoforms (FcRγIIIa, FcRγIIIb) on neutrophils by trastuzumab due to genetic polymorphism and heterogeneity in Asian SDC patients would have resulted in neutropenia and reported toxicities as compared to Caucasian breast cancer patients, it is an absolute possibility.Citation6
The amino acid sequence of clinical trastuzumab immunoglobulin G1 Fc (IgG1-Fc) contains lysine (L) 234-lysine (L) 235 (LL or wild-type) and asparagine (N) 297, proline (P) 329 in hinge and CH2 domains, respectively (http://www.imgt.org/3Dstructure-DB/cgi/details.cgi?pdbcode=7637). LL and N297 residues are critical to engage FcRγIII (CD16) receptors () and components of complement to activate both ADCC and complement-mediated cytotoxicity (CDC).Citation7 Although involvement of CDC remains to be established as a working mechanism of trastuzumab, ADCC and ADCP are known to play cytotoxic role in vitro and in vivo in breast cancer model studies.Citation4
Figure 1. Improving the safety of trastuzumab and docetaxel combinations by limiting Fc-effector functions. (a) Schematic of clinical trastuzumab fragment crystallizable (Fc) region. Hinge domain of trastuzumab Fc has lysine (L) residues at positions 234, 235 and CH2 domain of Fc contains glycosylation site (asparagine: N) at 297 and proline (P) at 329 positions, respectively. This configuration is named as trastuzumab (LL-PN). (b) In addition to antibody directed cell cytotoxicity (ADCC) activating cluster of differentiation 16a (CD16a, also known as FcRγIIIa) receptor on natural killer (NK) cells, LL-PN Fc residues of trastuzumab have potential to engage high affinity CD16b isoform (FcγRIIIb) receptor on neutrophils (in Asian population) resulting in secondary and nonspecific engagement and toxicity to neutrophils. (c) Schematic of modified trastuzumab fragment crystallizable region (Fc) region. Hinge domain of Fc has 234 and 235 lysine’s (L) replaced with alanine (A) residues and CH2 domain of Fc contains asparagine (N) mutated to glycine (G). Proline (P) at position 329 remains unchanged. This configuration is named as trastuzumab (LALA-PG). (d) LALA-PG mutations in trastuzumab Fc will limit its ability to effectively engage FcRγIIIa and potentially FcRγIIIb receptors on neutrophils in Asian population.
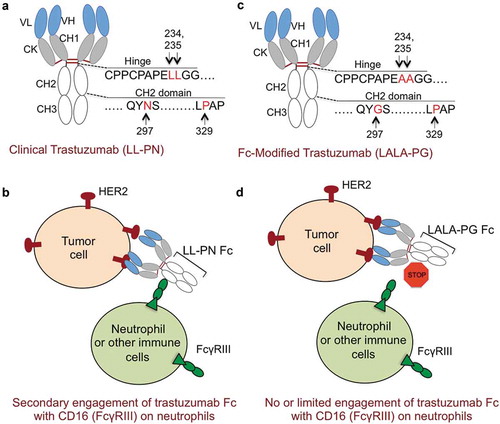
Recent studies using both mouse and human models have demonstrated that L234A-L235A, N297G, and P329 (LALA-PG) Fc-mutant IgG1 antibodies retain improved expression, stability in monospecific and bispecific formats, similar to wild-type antibodiesCitation7,Citation8 (). Importantly, LALA-PG mutant antibodies have significantly reduced binding to various complement components (C1q, C3) and various Fc receptors isoforms (FcRγI, FcRγII, FcRγIII and FcRγIV) on immune cells.Citation7 As neutrophils FcR’s might be involved in engaging trastuzumab-Fc to produce secondary autoimmune neutropenia (AIN), it is rationally anticipated that LALA-PG mutant version of trastuzumab will limit the unnecessary engagement neutrophils (). Nonetheless, the clinical trials (LALA-PG vs LL-NG trastuzumab) will define the fine balance between efficacy and safety (limited or no neutropenia) against Asian SDC patients.
Moreover, an important “trastuzumab only” treated patient group was not included in the clinical trial. Although taxane derivatives in chemotherapies are known to stimulate immune system, with non-inclusion of “trastuzumab only” patient group, it is difficult to attribute reported toxicities only to chemotherapy (docetaxel).Citation9 On the similar lines, would a discriminating presence of Akkermansia muciniphila or a distant gram-negative gut bacterial stain in Asian patients (but not in Caucasian patients) account for adverse effects of grade-4 neutropenia, is also a potential possibility.Citation10 The importance of gut microbiota and fluctuating immune response by clinical antibodies that engage and activate immune effector cells has been strongly supported using murine animal models and cancer patients.Citation10
Nonetheless, considering the highly positive efficacy of the combinatorial therapy,Citation1 it is also worth testing in a randomized and larger patients size clinical trial if addition of recombinant granulocyte colony-stimulating factor (G-CSF), which is known to reverse neutropenia, will reduce grade-3 and grade-4 toxicities in SDC patents. Finally, if the described toxicities are mainly due to docetaxel, trastuzumab-emtansine ADC is an alternate option for future clinical trials either as a single agent or in combination of reduced docetaxel dose (30 mg/m2 instead of 70 mg/m2) against in HER2+ SDC patients.
References
- Takahashi H, Tada Y, Saotome T, Akazawa K, Ojiri H, Fushimi C, Masubuchi T, Matsuki T, Tani K, Osamura RY, et al. Phase II trial of trastuzumab and docetaxel in patients with human epidermal growth factor receptor 2-positive salivary duct Carcinoma. J Clin Oncol. 2018;JCO1800545. doi:10.1200/JCO.18.00545.
- Martin-Castillo B, Pernas S, Dorca J, Álvarez I, Martínez S, Pérez-Garcia JM, Batista-López N, Rodríguez-Sánchez CA, Amillano K, Domínguez S, et al. A phase 2 trial of neoadjuvant metformin in combination with trastuzumab and chemotherapy in women with early HER2-positive breast cancer: the METTEN study. Oncotarget. 2018;9(35687–35704). doi:10.18632/oncotarget.26286
- Baselga J, Cortés J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(109–119). doi:10.1056/NEJMoa1113216
- Petricevic B, Laengle J, Singer J, Sachet M, Fazekas J, Steger G, Bartsch R, Jensen-Jarolim E, Bergmann M. Trastuzumab mediates antibody-dependent cell-mediated cytotoxicity and phagocytosis to the same extent in both adjuvant and metastatic HER2/neu breast cancer patients. J Transl Med. 2013;11(307). doi:10.1186/1479-5876-11-307
- Mok MY, Li WL. Do Asian patients have worse lupus? Lupus. 2010;19:1384–1390. doi:10.1177/0961203310375832.
- Mellor JD, Brown MP, Irving HR, Zalcberg JR, Dobrovic A. A critical review of the role of Fc gamma receptor polymorphisms in the response to monoclonal antibodies in cancer. J Hematol Oncol. 2013;6(1). doi:10.1186/1756-8722-6-1.
- Lo M, Kim HS, Tong RK, Bainbridge TW, Vernes JM, Zhang Y, Lin YL, Chung S, Dennis MS, Zuchero YJY, et al. Effector-attenuating substitutions that maintain antibody stability and reduce toxicity in mice. J Biol Chem. 2017;292(3900–3908). doi:10.1074/jbc.M116.767749
- Shivange G, Urbanek K, Przanowski P, Perry JS, Jones J, Haggart R, Kostka C, Patki T, Stelow E, Petrova Y, et al. A single-agent dual-specificity targeting of FOLR1 and DR5 as an effective strategy for ovarian cancer. Cancer Cell. 2018;34:331–345, E311. doi:10.1016/j.ccell.2018.07.005.
- Tsavaris N, Kosmas C, Vadiaka M, Kanelopoulos P, Boulamatsis D. Immune changes in patients with advanced breast cancer undergoing chemotherapy with taxanes. Br J Cancer. 2002;87(21–27). doi:10.1038/sj.bjc.6600347.
- Routy B, Le Chatelier E, Derosa L., Duong CP, Alou MT, Daillère R, Fluckiger A, Messaoudene M., Rauber C, Roberti MP, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359(91–97). doi:10.1126/science.aan3706
