ABSTRACT
How endothelial cells (ECs) survive in inflammatory environments remains unclear. We recently showed that TAK1 protects ECs against apoptosis induced by TNFα under physiological conditions in the intestine and liver, and under inflammatory conditions in other organs. Our results document that a single gene can affect cell fate decisions in mature ECs.
Endothelial cells (ECs) line the inner surface of blood vessels, forming quiescent monolayers in adults. However, they are not dormant but participate actively in basic functions, such as exchange of essential molecules between the blood and tissue, control of the vascular tone, prevention of blood clots and regulation of inflammation.Citation1,Citation2 Resting ECs do not interact with leukocytes, but once inflammation occurs, leukocytes can adhere to “activated” ECs, which supports their extravasation into inflamed tissues. Tumor necrosis factor-α (TNFα) is a major proinflammatory cytokine which activates EC and promotes adhesion of leukocytes by triggering transcription of adhesion molecule genes through the nuclear factor-kappa B (NF-κB) signaling pathway.Citation1 TNFα is a pleiotropic cytokine that can also trigger cell death;Citation3 but ECs are uniquely resistant to this effect under inflammatory conditions. In a recent study, we focused on mitogen-activated protein kinase kinase kinase 7 (MAP3K7), best known as TGF-β-activated kinase-1 (TAK1) as a key regulator of cell survival and death in ECs of adult mice. TAK1 is a member of the MAP3K family and is activated by diverse signaling molecules including TNFα binding to tumor necrosis factor receptor-1 (TNFR1).Citation4 Both systemic and Tie2-cre induced Tak1-knockout mice exhibit embryonic lethality due to vascular abnormalities, indicating that TAK1 is an important regulator of vascular development.Citation4,Citation5 However, the role of TAK1 in adult ECs remained unclear.
In our recent study,Citation6 we found that TAK1 is highly expressed in ECs of adult mice and to explore its function, we generated tamoxifen-inducible EC-specific TAK1-knockout mice by crossing VE-cadherin(BAC)-CreERT2 with Tak1flox mice (TAK1iEC-KO mice).Citation7,Citation8 To our surprise, although administration of tamoxifen to adult TAK1iEC-KO mice did result in hemorrhaging, this was limited to the intestine and liver. This was due to EC apoptosis, leading to severe anemia and rapid death of the animals within 11 days. Immunostaining of the liver of TAK1iEC-KO mice showed that endothelial TAK1 deficiency causes sequential changes to the structure of blood vessels, beginning with apoptosis of the EC, leaving only the vessel basement membrane which upon its collapse causes hemorrhage and tissue hypoxia. Using in vitro primary EC culture assays, we found that neutralization of TNFα can prevent EC apoptosis induced by TAK1 deficiency. Importantly, this TNFα-induced EC apoptosis was receptor interacting protein kinase 1 (RIP1)-dependent and was not the result of NF-κB inhibition.
We next investigated the mechanism whereby EC apoptosis is limited to the vasculature of the intestine and liver, but not other tissues, by focusing on the expression of TNFα. Interestingly, we found that CD11b+ cells responding to intestinal microbiota produce TNFα in the intestinal mucosa of wild-type mice. Elimination of these bacteria with a cocktail of antibiotics reduced the destruction of intestinal and liver blood vessels in TAK1iEC-KO mice, and hence prolonged survival. The liver harbors no TNFα-expressing cells in the non-inflamed steady state, but intestinal inflammation transduces inflammatory signals to the liver in some manner, triggering the activation of Kupffer cells which then secrete TNFα and induce EC apoptosis. These results suggest that TAK1 protects intestinal and liver ECs against cell death due to the intestinal microbiota under physiological conditions. In other words, this is one of the mechanisms which ensures host–bacterial symbiosis.
Because TAK1 is expressed in ECs of all organs examined, we further explored the role of this protein in different organs which do not show apparent vascular defects under physiological conditions. To this end, we induced acute inflammation in the lung or lower limb muscle by applying lipopolysaccharide or snake venom cardiotoxin respectively, and found that TNFα secretion associated with inflammation also induced vascular defects in these inflamed organs in TAK1iEC-KO mice. These results suggest that ECs are constantly prepared to protect themselves against sudden inflammation onset by means of constitutive TAK1 expression. Moreover, our results document that a single gene can affect fate decisions between survival and cell death in mature ECs under inflammatory conditions.
It is now clear that inflammation is a critical characteristic of the tumor microenvironment.Citation9 Therefore, we further asked whether TNFα-mediated EC apoptosis induced by TAK1 deficiency can be utilized as a therapeutic tool to destroy the tumor vasculature. In the tumor, it is reported that TNFα induces angiogenesis.Citation10 However, when endothelial TAK1 is inhibited, TNFα induced a massive amount of apoptosis of tumor ECs and destroyed tumor blood vessels by a mechanism different from the vascular endothelial growth factor (VEGF)-VEGF receptor signaling pathway inhibition commonly adopted clinically. Moreover, extensive destruction of the tumor vasculature induced growth arrest and apoptosis of tumor parenchymal cells, resulting in severe necrosis and regression of the tumor.
In conclusion, our work has identified mechanisms ensuring survival and maintenance of homeostasis of ECs in the intestine and liver under physiological conditions and in other organs under inflammatory conditions (). Deficiency of a single gene in mature EC resulting in the destruction of the vasculature and rapid death of the animal is very rare. Modulation of the TAK1 signaling pathway which induces EC death upon TNFα stimulation may be a good target for anti-angiogenic therapy in the future. Further work will be required to clarify the precise signaling pathways of cell death in ECs.
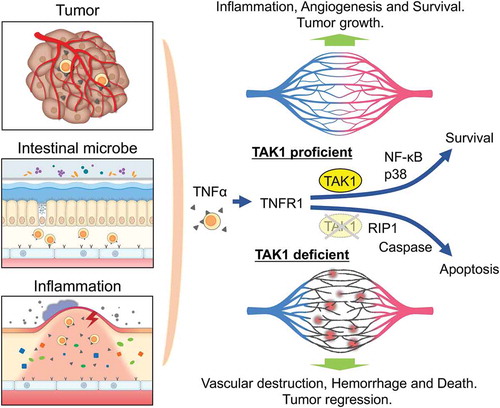
Figure 1. Role of TAK1 in maintaining vascular integrity by preventing endothelial cells apoptosis caused by TNFα secreted in the microenvironments.
TNFα produced in the tumor microenvironment, induced by gut microbe activity, or induced by inflammation, stimulates through TNFR1 on endothelial cells (ECs). In wild-type mice, TNFR1 activation leads to NF-κB and p38 activation which induces cell activation and enhances survival. However, in the TAK1-deficient ECs, apoptosis occurs in a RIP1-dependent manner, which resulting in vascular destruction, hemorrhage and death of the animal. Targeting endothelial TAK1 may be developed as an anti-angiogenic therapy. TAK1, TGF-β-activated kinase-1; TNFα, tumor necrosis factor alpha; TNFR1, tumor necrosis factor receptor 1; NF-κB, nuclear factor-kappa B; RIP1, receptor interacting protein kinase 1.
Acknowledgments
We thank Ms. M. Ishida, Ms. N. Fujimoto, and Ms. Y. Mori for assistance.
Disclosure Statement
The authors declare no competing interests.
Additional information
Funding
References
- Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7(10):803–815. doi:10.1038/nri2171.
- Pober JS, Min W, Bradley JR. Mechanisms of endothelial dysfunction, injury, and death. Annu Rev Pathol. 2009;4:71–95. doi:10.1146/annurev.pathol.4.110807.092155.
- Annibaldi A, Meier P. Checkpoints in TNF-induced cell death: implications in inflammation and cancer. Trends Mol Med. 2018;24(1):49–65. doi:10.1016/j.molmed.2017.11.002.
- Morioka S, Inagaki M, Komatsu Y, Mishina Y, Matsumoto K, Ninomiya-Tsuji J. TAK1 kinase signaling regulates embryonic angiogenesis by modulating endothelial cell survival and migration. Blood. 2012;120(18):3846–3857. PMID:3488895. doi:10.1182/blood-2012-03-416198
- Jadrich JL, O’Connor MB, Coucouvanis E. The TGF beta activated kinase TAK1 regulates vascular development in vivo. Development. 2006;133(8):1529–1541. doi:10.1242/dev.02333.
- Naito H, Iba T, Wakabayashi T, Tai-Nagara I, Suehiro JI, Jia W, Eino D, Sakimoto S, Muramatsu F, Kidoya H, et al. TAK1 prevents endothelial apoptosis and maintains vascular integrity. Dev Cell. 2019;48(2):151–166e7. doi:10.1016/j.devcel.2018.12.002.
- Okabe K, Kobayashi S, Yamada T, Kurihara T, Tai-Nagara I, Miyamoto T, Mukouyama YS, Sato TN, Suda T, Ema M, et al. Neurons limit angiogenesis by titrating VEGF in retina. Cell. 2014;159(3):584–596. doi:10.1016/j.cell.2014.09.025.
- Sato S, Sanjo H, Takeda K, Ninomiya-Tsuji J, Yamamoto M, Kawai T, Matsumoto K, Takeuchi O, Akira S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat Immunol. 2005;6(11):1087–1095. doi:10.1038/ni1255.
- Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. PMID:PMC2803035. doi:10.1038/nature01322
- Leibovich SJ, Polverini PJ, Shepard HM, Wiseman DM, Shively V, Nuseir N. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature. 1987;329(6140):630–632. doi:10.1038/329630a0.
