ABSTRACT
Alterations of cell mechano-environment and metabolism are common features of malignant neoplasm. We recently showed that increased stiffness of extracellular matrix is intrinsically linked to up-regulation of proline synthesis through a mechano-responsive fermitin family homolog 2 (FERMT2, best known as kindlin-2) and pyrroline-5-carboxylate reductase 1(PYCR1) complex, which in turn promotes collagen matrix synthesis, cell proliferation, survival, and cancer progression.
The interplay between cell mechano-environment and metabolism is critically involved in cancer progression. It has been well recognized, for example, that increased extracellular matrix (ECM) stiffness is intimately associated with tumorigenesis and progression. ECM stiffening not only is the result of tumor fibrosis, and growth (and therefore is widely used for diagnosis of a tumor in various organs) but also functionally contributes to cancer progression. Metabolic reprograming is another common feature of cancer, which allows cancer cells to proliferate, survive and disseminate under altered microenvironment. Among amino acids, the demand for proline is in particular increased in cancer cells.Citation1 Proline metabolism is important for energy production, redox balance and protein synthesis, in particular, that of collagen, as approximately 25% of amino acids incorporated into collagen are proline.Citation2 Indeed, it has been shown that pyrroline-5-carboxylate reductase 1 (PYCR1), a key enzyme in proline synthesis, is one of the most overexpressed metabolic enzymes in cancer.Citation2 In a recent study, we have found that these two common features of malignant neoplasm (i.e., increases of ECM stiffness and proline synthesis) are intrinsically linked through a mechano-responsive fermitin family homolog 2 (FERMT2, best known as kindlin-2) -PYCR1 protein complex.Citation3
Kindlin-2 is a widely expressed component of cell-ECM adhesions.Citation4 While the functions of kindlin-2 in integrin-mediated cell-ECM adhesion and signaling have been well recognized,Citation4–Citation7 its role in metabolic pathways was unknown. Initial evidence for a role of kindlin-2 in proline metabolism was from a nanoscale liquid chromatography coupled to tandem mass spectrometry screen, in which we found that kindlin-2 physically associated with PYCR1.Citation3 Using recombinant kindlin-2 and PYCR1 proteins, we confirmed that they directly interacted with each other. Furthermore, biochemical, confocal microscopic and fluorescence resonance energy transfer analyses revealed that kindlin-2 was localized in not only cell-ECM adhesions but also mitochondria where it formed a complex with PYCR1. Remarkedly, mechanical signals from cell environment (e.g., ECM stiffening) promoted kindlin-2 mitochondrial translocation and interaction with PYCR1, resulting in elevations of PYCR1 level, proline synthesis, and cell proliferation.Citation3 In the same study, we have also assessed whether kindlin-2 functions in the regulation of the levels of PYCR1, proline and collagen matrix and the growth of tumor in vivo. The results showed that the levels of both kindlin-2 and PYCR1 were markedly increased in human and mouse lung adenocarcinoma, which exhibited greater stiffness compared with that of healthy tissue regions adjacent to the tumors.Citation3 Importantly, ablation of Fermt2 from lung adenocarcinoma in mouse significantly reduced the levels of Pycr1, proline synthesis, and collagen matrix, resulting in marked inhibition of tumor growth and reduction of mortality rate.Citation3 Thus, kindlin-2 and PYCR1 appear to mediate a positive feedback system between cellular mechano-environment and proline synthesis, in which ECM stiffening promotes kindlin-2 interaction with PYCR1, resulting in elevated levels of PYCR1 and proline synthesis and consequently increases cell proliferation, survival, collagen matrix synthesis and tumor growth (). The elevation of collagen level in ECM, in turn, increases ECM stiffness, which further promotes kindlin-2 interaction with PYCR1 and cancer progression (). Ablation of Fermt2 disrupts this positive feedback system and therefore suppresses tumor growth in vivo.Citation3
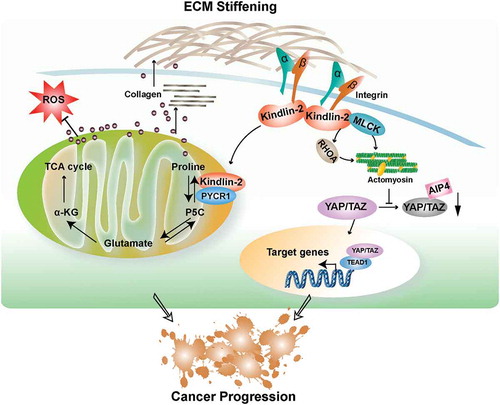
Figure 1. Kindlin-2-mediated signaling pathways that link mechano-environment to proline synthesis, YAP/TAZ signaling and cancer progression.
The figure depicts signaling pathways through which fermitin family homolog 2 (FERMT2, best known as kindlin-2) links mechano-environment to proline synthesis and Yes-associated protein (YAP)/WW domain containing transcription regulator 1 (WWTRl, best known as TAZ) signaling. Kindlin-2 is recruited to cell-extracellular matrix (ECM) adhesions through interaction with integrins, where it promotes integrin activation, clustering, and downstream signaling. ECM stiffening promotes kindlin-2 translocation to mitochondria, where it interacts with pyrroline-5-carboxylate reductase 1(PYCR1), resulting in elevations of PYCR1 level and proline synthesis that promotes cell proliferation and collagen matrix synthesis, which further increases ECM stiffness.Citation3 Kindlin-2-mediated regulation of proline synthesis also helps to maintain redox balance and cell survival. In addition, kindlin-2 interacts with myosin light chain kinase (MLCK) in response to ECM stiffening, which promotes myosin light chain phosphorylation and actomyosin contraction, resulting in inhibition of neural precursor cell expressed, developmentally down-regulated 4(NEED4)-like E3 ubiquitin ligase Atrophin-interacting Protein 4(AIP4)-mediated YAP/TAZ degradation and increase of YAP/TAZ expression and signaling.Citation8 α-KG: α-ketoglutarate; P5C: Δ1-pyrroline-5-carboxylate; ROS: reactive oxygen species; TCA: tricarboxylic acid cycle; TEAD1: TEA domain family member 1; RHOA: Ras homolog family member A.
While it is clear that kindlin-2 interaction with PYCR1 and regulation of proline metabolism are critically involved in tumor fibrosis and growth, kindlin-2 likely also participates in other aspects of mechanotransduction during cancer progression. For example, kindlin-2 is known to directly interact with integrin β cytoplasmic domains.Citation9 The binding of kindlin-2 to integrin is likely important for mechanical force-induced integrin activation and clusteringCitation7,Citation10 and consequently downstream signaling. Additionally, we recently found that kindlin-2 interacts with myosin light chain kinase (MLCK) and regulates myosin light chain phosphorylation and actomyosin contraction in response to mechanical signals from cellular microenvironment, which in turn regulates Yes-associated protein (YAP) and WW domain containing transcription regulator 1 (WWTRl, best known as TAZ) expression and signaling ().Citation8 Given the prominent roles of YAP/TAZ in cancer development and progression, kindlin-2-mediated regulation of YAP/TAZ expression and signaling may also contribute to mechano-regulation of gene expression and cancer progression. Thus, targeting the kindlin-2-mediated mechanotransduction pathways appears to be an attractive strategy for suppressing fibrosis and cancer progression.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Loayza-Puch F, Rooijers K, Buil LC, Zijlstra J, Oude Vrielink JF, Lopes R, Ugalde AP, van Breugel P, Hofland I, Wesseling J, et al. Tumour-specific proline vulnerability uncovered by differential ribosome codon reading. Nature. 2016;530(7591):490–494. doi:10.1038/nature16982.
- Phang JM, Liu W, Hancock CN, Fischer JW. Proline metabolism and cancer: emerging links to glutamine and collagen. Curr Opin Clin Nutr Metab Care. 2015;18(1):71–77. doi:10.1097/MCO.0000000000000121.
- Guo L, Cui C, Zhang K, Wang J, Wang Y, Lu Y, Chen K, Yuan J, Xiao G, Tang B, et al. Kindlin-2 links mechano-environment to proline synthesis and tumor growth. Nat Commun. 2019;10(1):845. doi:10.1038/s41467-019-08772-3.
- Tu Y, Wu S, Shi X, Chen K, Wu C. Migfilin and Mig-2 link focal adhesions to filamin and the actin cytoskeleton and function in cell shape modulation. Cell. 2003;113:37–47.
- Larjava H, Plow EF, Wu C. Kindlins: essential regulators of integrin signalling and cell-matrix adhesion. EMBO Rep. 2008;9(12):1203–1208. doi:10.1038/embor.2008.202.
- Plow EF, Qin J, Byzova T. Kindling the flame of integrin activation and function with kindlins. Curr Opin Hematol. 2009;16(5):323–328. doi:10.1097/MOH.0b013e32832ea389.
- Sun Z, Costell M, Fassler R. Integrin activation by talin, kindlin and mechanical forces. Nat Cell Biol. 2019;21(1):25–31. doi:10.1038/s41556-018-0234-9.
- Guo L, Cai T, Chen K, Wang R, Wang J, Cui C, Yuan J, Zhang K, Liu Z, Deng Y, et al. Kindlin-2 regulates mesenchymal stem cell differentiation through control of YAP1/TAZ. J Cell Biol. 2018;217(4):1431–1451. doi:10.1083/jcb.201612177.
- Shi X, Ma YQ, Tu Y, Chen K, Wu S, Fukuda K, Qin J, Plow EF, Wu C. The MIG-2/integrin interaction strengthens cell-matrix adhesion and modulates cell motility. J Biol Chem. 2007;282(28):20455–20466. doi:10.1074/jbc.M611680200.
- Li H, Deng Y, Sun K, Yang H, Liu J, Wang M, Zhang Z, Lin J, Wu C, Wei Z, et al. Structural basis of kindlin-mediated integrin recognition and activation. Proc Natl Acad Sci USA. 2017;114(35):9349–9354. doi:10.1073/pnas.1703064114.
