ABSTRACT
In our recent publication, we describe a mechanism by which peroxisomes are protected from degradation by autophagy under basal conditions. Taking a page from mitophagy, peroxisomes also recruit the mitochondria deubiquitinating enzyme USP30 to counter the action of PEX2, the peroxisomal E3 ubiquitin ligase to regulate pexophagy.
Selective autophagy is a critical process used by the cell to remove damaged or dysfunctional organelles. Even though selective autophagy is necessary for normal cellular function, and its dysregulation results in many human diseases,Citation1 surprisingly little is known about how selective autophagy of different substrates is regulated.
Peroxisomes are metabolic organelles responsible for, amongst other things, metabolizing fatty acids and scavenging reactive oxygen species.Citation2 However, dysregulation in peroxisome homeostasis results in a rare class of genetic disorders, Peroxisome Biogenesis Disorders (PBDs).Citation3 While the low peroxisome number and function was previously thought to be caused by reduced peroxisome biogenesis, a recent study has shown that the most common form of the disease is caused by increased degradation.Citation4 Regulation of pexophagy is therefore essential to prevent disease, and thus formed the basis of our study.Citation5
Peroxisomes, like mitochondria, are degraded through selective autophagy, termed pexophagy and mitophagy respectively. Pexophagy can be induced by various stimuli, but the focus of our study was amino acid starvation induced pexophagy. Amino acid starvation, through inhibition of mammalian target of rapamycin (mTOR), results in the stabilization of the E3 ligase PEX2 on peroxisome membranes.Citation6 PEX2 acts to ubiquitinate membrane proteins, resulting in peroxisomal degradation. This serves as the first regulatory step to ensure peroxisomes are not lost under basal conditions.
It has been shown that mitophagy, in addition to the regulation offered by recruitment of the E3 ligase Parkin to damaged organelles, is also negatively regulated by a deubiquitinase, ubiquitin specific peptidase (USP30).Citation7 Peroxisomes have not previously been shown to have a negative pexophagy regulator. In our previous study describing the role of USP30 at the mitochondria, we noticed small USP30-containing puncta that did not contain mitochondrial markers.Citation7 Interestingly, these puncta co-localized with peroxisomes, suggesting it may play a role in pexophagy. Our published study aimed to characterize the role of USP30 in peroxisome maintenance.Citation5
We began by showing the targeting of USP30 to peroxisomes, demonstrating that over-expressed wild type USP30 is co-localized with peroxisomes significantly more than the mitochondrial targeted TOM20-USP30. Using a stop-anchor PEX16 (saPEX16) which is ER retained, we showed that USP30 can be recruited to the ER by the peroxisome membrane importer PEX16, while mitochondrial targeting remained intact, suggesting that USP30 is targeted to the peroxisomes with the assistance of PEX16. We next found that over-expressed USP30 is able to inhibit peroxisome loss and stop pexophagy upon amino acid starvation. Interestingly, WT USP30 and peroxisome targeted PMP34-USP30, but not TOM20-USP30, was able to demonstrate this inhibition, suggesting that USP30 is acting directly at the peroxisome.
To determine whether UPS30 functions to prevent basal pexophagy, we used siRNA mediated knockdown to understand its role under basal conditions. We found that USP30 depletion resulted in increased autophagy dependent peroxisome loss. In addition, co-depletion of PEX2 resulted in an abolishment of the peroxisome loss seen in USP30 depleted cells, suggesting that USP30 acts in opposition to PEX2 and is required to keep basal pexophagy to a minimum. ())
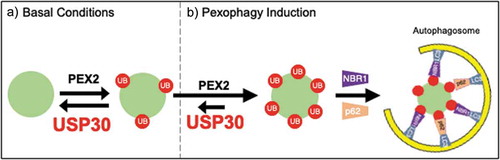
Figure 1. USP30 regulates basal pexophagy.
a) Under basal conditions, USP30 and PEX2 work in opposition to maintain peroxisome homeostasis. b) During pexophagy induction, USP30 can no longer maintain low peroxisome ubiquitination, autophagy receptors NBR1 and sequestosome 1 (p62) are recruited to facilitate engulfment by the autophagosome and peroxisomes are lost.
As a deubiquitinase, USP30 acts to remove ubiquitin moieties from proteins. Therefore, we wanted to determine what substrates are deubiquitinated by USP30. Using co-IP, we showed that both PEX5 and PMP70, two known targets of PEX2, are deubiquitinated in USP30 depleted cells. In addition to this, we found that over-expression of USP30 resulted in decreased recruitment of the ubiquitin binding autophagy receptor neighbor of BRCA1 gene 1 (NBR1) upon pexophagy induction, suggesting that USP30 acts to regulate basal pexophagy through deubiquitination of its substrates. ((b))
Previously, we have shown that the most common PBD mutation, PEX1 G843D, results in an accumulation of ubiquitinated PEX5 on the peroxisome membrane, resulting in peroxisome loss through increased pexophagy.Citation4 To test whether USP30 can prevent pexophagy in this background we overexpressed USP30 in a PEX1 G843D PBD cell line and found that it can rescue the loss of peroxisomes, suggesting a potential therapeutic approach in combatting this disease.
During the revision of our manuscript, a study was published by Sylvie Urbé’s group demonstrating that endogenous USP30 localizes to the peroxisome where it regulates basal pexophagy.Citation8 Our work adds to their work by the identification of the E3 ligase, and at least two of UPS30’s substrate. Together, these studies demonstrate the novel role of USP30 in pexophagy.
Many questions still remain unanswered. First and foremost, why would the cell have a single regulator for both pexophagy and mitophagy. This gives rise to the question of targeting and regulation. Peroxisomes and mitochondria are degraded under different conditions, one example being amino acid starvation which induces pexophagy while protecting mitochondria.Citation9 Is there any condition, therefore, that would induce an upregulation of USP30, or a change in its cellular distribution, to allow for degradation of one organelle over the other? We and Urbé’s group have shown that USP30 is targeted to the mitochondria and peroxisome independently. Is this difference in targeting a mechanism by which USP30 differentially regulates these two organelles? Or is USP30 differentially regulated on its target organelle?
Finally, we’ve shown that USP30 is able to increase peroxisomes in PEX1 G843D fibroblasts, suggesting a potential therapeutic approach in disease. While continuing to evaluate this potential, we would ask whether USP30 could be used therapeutically in other disease models. Peroxisomes, as metabolic regulators, have been implicated in many types of cancer, and neurodegenerative disorders.Citation2,Citation10 Studying pexophagy regulation may provide novel insights to understanding the pathophysiology and new therapeutic strategies to various diseases.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- Anding AL, Baehrecke EH. Developmental cell cleaning house: selective autophagy of organelles. Dev Cell. 2017;41:10–22. doi:10.1016/j.devcel.2017.02.016.
- Berger J, Dorninger F, Forss-Petter S, Kunze M. Peroxisomes in brain development and function. Biochim Biophys Acta - Mol Cell Res. 2016;1863(5):934–955. doi:10.1016/j.bbamcr.2015.12.005.
- Braverman NE, D’Agostino MD, MacLean GE. Peroxisome biogenesis disorders: biological, clinical and pathophysiological perspectives. Dev Disabil Res Rev. 2013;17(3):187–196. doi:10.1002/ddrr.1113.
- Law KB, Bronte-Tinkew D, Di Pietro E, Snowden A, Jones RO, Moser A, Brumell LH, Braverman N, Kim PK. The peroxisomal AAA ATPase complex prevents pexophagy and development of peroxisome biogenesis disorders. Autophagy 2017;13(5):868–884. doi:10.1080/15548627.2017.1291470.
- Riccio V, Demers N, Hua R, Vissa M, Cheng DT, Strilchuk AW, Wang Y, McQuibban GA, Kim PK. Deubiquitinating enzyme USP30 maintains basal peroxisome abundance by regulating pexophagy. J Cell Biol. 2019;218:798–807. doi:10.1083/jcb.201804172.
- Sargent G, Zutphen T, Van, Shatseva T, Zhang L, Di Giovanni V, Bandsma R, Kim PK. PEX2 is the E3 ubiquitin ligase required for pexophagy during starvation. J Cell Biol. 2016;214(6):677–690. doi:10.1083/jcb.201511034.
- Wang Y, Serricchio M, Jauregui M, Shanbhag R, Stoltz T, Di Paolo CT, Kim PK, McQuibban GA. Deubiquitinating enzymes regulate PARK2-mediated mitophagy. Autophagy. 2015;11(4):595–606. doi:10.1080/15548627.2015.1034408.
- Marcassa E, Kallinos A, Jardine J, Rusilowicz‐Jones EV, Martinez A, Kuehl S, Islinger M, Clague MJ, Urbé S. Dual role of USP30 in controlling basal pexophagy and mitophagy. EMBO Rep. 2018;19(7):e45595. doi:10.15252/embr.201745595.
- Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci. 2011;108(25):10190–10195. doi:10.1073/pnas.1107402108.
- Dahabieh MS, Di Pietro E, Jangal M, Goncalves C, Witcher M, Braverman NE, Del Rincón SV. Peroxisomes and cancer: the role of a metabolic specialist in a disease of aberrant metabolism. Biochim Biophys Acta - Rev Cancer. 2018;1870(1):103–121. doi:10.1016/j.bbcan.2018.07.004.
