ABSTRACT
Both autophagy and hERG1 potassium channels have been shown to promote tumor progression and resistance to treatment. Our findings indicate that the antibiotic clarithromycin can target hERG1 and modulate autophagy to promote the death of chemoresistant colorectal cancer cells. Thus, clarithromycin stands out as promising combinatorial partner to improve the efficacy of chemotherapy in patients with colorectal cancer.
Autophagy is an evolutionarily conserved catabolic cellular process that allows the recycling of metabolic precursors. Autophagy involves the sequential sequestration and digestion of cytosolic entities (such as, senescent, damaged or potentially harmful organelles, and proteins) through the formation of double-membrane organelles (autophagosomes) which ultimately fuse with lysosomes to form degradation-competent autolysosomes.Citation1,Citation2 In mammalian cells, autophagy is essential for basal intracellular homeostasis and it is primarily used as cytoprotective response during nutrient deprivation or metabolic stress. However, alterations in the autophagic signaling pathway have been associated with many human diseases, including cancer. Several studies suggest that autophagy can play diverse roles in the oncological settings, largely depending on cellular context and tumor stage. Thus, while autophagy generally suppresses the transformation of healthy cells by resolving cellular damage, malignant cells can harness autophagy to survive microenvironmental stress, progress, and disseminate despite therapy. Moreover, autophagy also influences responses to danger signals consequent to therapy-induced immunogenic cell death and anticancer immunosurveillance.Citation1,Citation2 For these reasons, over the past two decades, considerable efforts have been devoted to the development of autophagy modulators for the treatment of cancer. However, such efforts have been largely disappointing, in particular reflecting the intrinsically limited specificity of clinically available drugs for the autophagic process or for cancer cells, resulting in pleiotropic effects.Citation3 In this context, the precise understanding of the biochemical features of autophagy as a tumor-promoting process and the identification of cancer-cell specific biomarkers of autophagic flux, might support the development of increasingly specific and effective autophagy-targeting drugs.
We initially set to test the impact of clarithromycin (CLA) on the autophagic flux of human colorectal cancer (CRC) cells.Citation4 CLA is a member of macrolide antibiotics that had previously been reported to inhibit autophagy in different preclinical cancer models, but not CRC.Citation5 Moreover, in mammalian cells, CLA has been reported to target potassium voltage-gated channel subfamily H member 2 (KCNH2) a K+ channel also known as hERG1 that is aberrantly expressed in different human tumors, including CRC.Citation6–Citation8
CLA demonstrated anti-proliferative activity in vitro at concentrations ≥80 μM in human hERG1+ CRC cells (HCT-116, HT-29 and LS174 T cells), linked to a dual effect on autophagy modulation and initiation of apoptosis. We observed an initial (24 h after stimulation) activation of autophagy by CLA, as suggested by (1) an increase in acidic vesicular organelles (AVOs) in the cytosol; (2) the accumulation of the lipidated form of microtubule-associated protein 1 light chain 3 beta (MAP1LC3B, best known as LC3-II), and (3) the degradation of the autophagic cargo receptor sequestome 1 (SQSTM1, best known as p62),Citation4 which are common markers for autophagy.Citation2 After prolonged exposure to CLA (48 h), autophagic vacuoles were characterized by increased size and decreased acidity, paralleled by decreased LC3-II and increased p62 levels, suggestive of a late inhibition of the autophagic flux. This late impairment of the autophagic process culminated with G2/M arrest and p53-dependent, caspase-accelerated apoptotic cell death.Citation4
The mechanism by which CLA interferes with autophagy has not been fully elucidated.Citation5 We thus studied the involvement of hERG1, finding that KCNH2 deletion abrogated CLA effects on CRC cells. Consistently, hERG1 overexpression sensitized HEK-293 cells (which normally lack hERG1 expression) to CLA. Immunoprecipitation experiments showed that hERG1 binds the p85 subunit of the phosphoinositide-3-kinase (PI3K) complexes and that CLA inhibits such association, resulting in limited AKT serine/threonine kinase 1 (AKT1), mitogen-activated protein kinase 3 (MAPK3, best known as ERK1) and MAPK1 (best known as ERK2) phosphorylation and consequent autophagy modulation. Transfecting HCT-116 cells with plasmids with different hERG1 mutants and inhibiting hERG1 currents with specific pharmacological agents, also demonstrated that the effect of CLA on HCT-116 cells is independent on hERG1 conduction and is favored when hERG1 resides in a closed conformation.Citation4
We finally set to test the potential of CLA to overcome cytoprotective autophagy induced by 5-fluorouracil (5-FU, a common chemotherapeutic for CRC), in preclinical (in vitro and in vivo) models of human CRC.Citation4 The efficacy of 5-FU against CRC cells is indeed limited by autophagy upregulation.Citation9 We found that CLA significantly enhances the pro-apoptotic effect of 5-FU on HCT-116 cells maintained in vitro, both as monolayers and spheroids, as well as in HCT-116 xenografts established in immunodeficient mice. This synergistic effect was paralleled by reduced hERG1 expression and ERK1/2 phosphorylation, increased p53 expression and caspase activation.Citation4
In summary, our results suggest that CLA modulates autophagy by targeting the ability of hERG1 to bind PI3K, which can be exploited to minimize chemoresistance in models of CRC (). Our findings may have important implications for the development of optimally personalized treatments since hERG1 could be used as biomarker to select those patients most likely to benefit from autophagy inhibition.
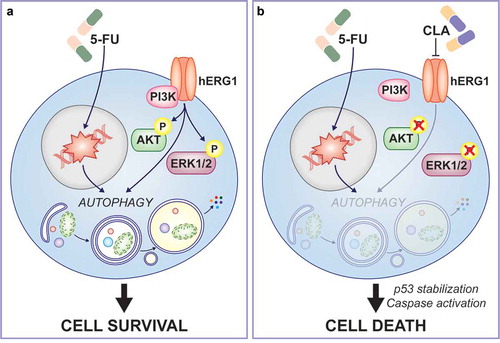
Figure 1. Cellular and molecular mechanisms of clarithromycin anti-cancer effects. (a) In human colorectal cancer (CRC) cells the potassium voltage-gated channel subfamily H member 2 (KCNH2), best known as hERG1, binds to the p85 subunit of the phosphoinositide-3-kinase (PI3K) complexes promoting AKT serine/threonine kinase 1 (AKT1) and mitogen-activated protein kinase 3 (MAPK3, best known as ERK1) and MAPK1 (best known as ERK2) phosphorylation and tumor proliferation. 5-florouracil (5-FU) efficacy is limited by the induction of cytoprotective autophagy in CRC cells. (b) Pharmacological inhibition of the hERG1/PI3K complex formation by clarithromycin (CLA) results in limited AKT and ERK1/2 phosphorylation and consequent autophagy modulation, which in turn induces p53-dependent, caspase-accelerated apoptotic cell death and minimizes resistance to 5-FU in human CRC cells.
Although CLA is traditionally used for many types of bacterial infections, our findings point to CLA as a promising combinatorial partner for chemotherapy. Early clinical trials testing CLA in patients with hematological neoplasms demonstrated that this approach is safe and associated with clinical benefits.Citation5 Although induction of protective autophagy represents a major challenge in cancer therapy, autophagy inhibition can affect anti-tumor immune responses and cardiovascular homeostasis.Citation2,Citation10 Since the effects of CLA on malignant cells stem from the dissociation of hERG1/PI3K complexes that are not found in normal cell types, our findings suggest that CLA may constitute an innovative tool to inhibit autophagy in cancer cells in the absence of overt immunosuppressive and cardiovascular toxicity. Additional preclinical and clinical testing is required to elucidate this possibility.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
GP is supported by the 2019 Laura Ziskin Prize in Translational Research (#ZP-6177) from the Stand Up to Cancer (SU2C) initiative (PI: Formenti).
References
- Rybstein MD, Bravo-San Pedro JM, Kroemer G, Galluzzi L. The autophagic network and cancer. Nat Cell Biol. 2018;20(3):1–3. doi:10.1038/s41556-018-0042-2.
- Galluzzi L, Green DR. Autophagy-independent functions of the autophagy machinery. Cell. 2019;177(7):1682–1699. doi:10.1016/j.cell.2019.05.026.
- Levy JMM, Towers CG, Thorburn A. Targeting autophagy in cancer. Nat Rev Cancer. 2017;17(9):528–542. doi:10.1038/nrc.2017.53.
- Petroni G, Bagni G, Iorio J, Duranti C, Lottini T, Stefanini M, Kragol G, Becchetti A, Arcangeli A. Clarithromycin inhibits autophagy in colorectal cancer by regulating the hERG1 potassium channel interaction with PI3K. Cell Death Dis. 2020;11(3):161. doi:10.1038/s41419-020-2349-8.
- Van Nuffel AM, Sukhatme V, Pantziarka P, Meheus L, Sukhatme VP, Bouche G. Repurposing drugs in oncology (ReDO)-clarithromycin as an anti-cancer agent. Ecancermedicalscience. 2015;9:513. doi:10.3332/ecancer.2015.513.
- Stanat SJ, Carlton CG, Crumb WJ Jr, Agrawal KC, Clarkson CW. Characterization of the inhibitory effects of erythromycin and clarithromycin on the HERG potassium channel. Mol Cell Biochem. 2003;254(1/2):1–7. doi:10.1023/A:1027309703313.
- Muratori L, Petroni G, Antonuzzo L, Boni L, Iorio J, Lastraioli E, Bartoli G, Messerini L, Di Costanzo F, Arcangeli A. hERG1 positivity and Glut-1 negativity identifies high-risk TNM stage I and II colorectal cancer patients, regardless of adjuvant chemotherapy. Onco Targets Ther. 2016;9:6325–6332. doi:10.2147/OTT.
- Becchetti A, Petroni G, Arcangeli A. Ion channel conformations regulate integrin-dependent signaling. Trends Cell Biol. 2019;29(4):298–307. doi:10.1016/j.tcb.2018.12.005.
- Park JM, Huang S, Wu TT, Foster NR, Sinicrope FA. Prognostic impact of Beclin 1, p62/sequestosome 1 and LC3 protein expression in colon carcinomas from patients receiving 5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther. 2013;14(2):100–107. doi:10.4161/cbt.22954.
- Bravo-San Pedro JM, Kroemer G, Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ Res. 2017;120(11):1812–1824. doi:10.1161/CIRCRESAHA.117.311082.
