ABSTRACT
Endocytic trafficking has emerged as an essential mechanism to spatiotemporally coordinate signaling protein complexes that control cytoskeletal dynamics and cell motility. Our study established an unexpected regulatory mechanism whereby ADP ribosylation factors 6 (ARF6) controls the stability and endosomal localization of RAS homologous protein B (RHOB) to regulate cell invasion downstream of the oncogenic receptor tyrosine kinase, MET.
Article
Endocytosis and recycling are complex and ligand dependent mechanisms that regulate extracellular signals, directly influencing the localization, duration, and intensity of signaling outputs to exert temporal and spatial control of signal transduction. Through the uptake and processing of various cargoes, ranging from small molecules to activated signaling receptors and protein complexes, endocytosis and recycling also regulate multiple biological processes associated with both physiological and tumorigenic cellular states.Citation1 The MET receptor tyrosine kinase (RTK), in response to its ligand, hepatocyte growth factor (HGF), coordinates actin cytoskeletal remodeling and the formation and turnover of focal adhesions (FAs).Citation2 Dysregulated MET signaling contributes to tumorigenesis by supporting cell proliferation and survival, as well as promoting epithelial remodeling, cell scattering and invasion.Citation2,Citation3 The detailed mechanisms through which MET regulates cancer cell invasion and cytoskeletal dynamics are, however, still poorly understood.
The ADP ribosylation factors (ARF) and RAS homologous protein (RHO) GTPase subfamilies exert important functions in endocytic trafficking. The small GTPase ARF6 regulates cytoskeletal organization, membrane trafficking, and endocytic recycling in concert with various effectors. ARF6 localizes to the plasma membrane (PM) and endosomal compartments and can be activated downstream of the MET RTK.Citation4 ARF6 also contributes to endocytic trafficking of MET into RAB4-positive tubular domains of the early endosome, enhancing MET recycling.Citation5 The RHO GTPases are crucial regulators of actin cytoskeletal rearrangements and FA dynamics which are important for cell motility.Citation6 Although there are over 20 human RHO GTPases, few have been studied downstream of MET. Among these small GTPases, the RHO subfamily (RHOA, RHOB, and RHOC) shares more than 85% amino acid sequence identity, with most differences concentrated in the C-terminus. Strikingly, in contrast to RHOA and RHOC, which are 91% identical in sequence, RHOB localizes not only at the PM and the cytoplasm, but also on endosomes and multivesicular bodies. The localization of RHOB to endosomes is well documented,Citation7–Citation9 however the pathways downstream of this signaling protein and the mechanistic details of its retention and role at endosomal membranes remained poorly understood.
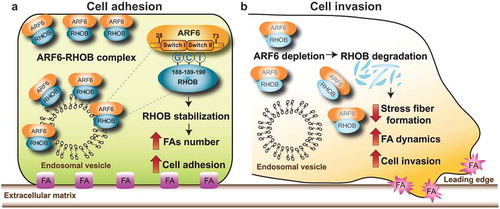
In Zaoui et al,Citation10 we interrogated the role of ARF6 in the endocytic trafficking of RHOB downstream from the MET RTK. We discovered that ARF6 directly interacts with RHOB and that this interaction is critical for RHOB localization to endosomes as well as RHOB stability. Notably, depletion of ARF6 results in loss of RHOB from the endosomal compartments and leads to RHOB degradation through an endo-lysosomal pathway. A unique feature of RHOB, which is not found in other RHO GTPases, is the tripeptide “GCI” (glycine, cysteine, and, isoleucine) at residues 188–190, localized in the hypervariable C-terminus domain.Citation9 Notably, we established that the GCI tripeptide is required for ARF6-RHOB interaction, as insertion of this GCI peptide into RHOA (RHOA-GCI) was sufficient to confer ARF6 interaction to RHOA.Citation10
Since FAs link actin stress fibers to the extracellular matrix (ECM) and both ARF and RHO families can regulate cell adhesion by enhancing actomyosin contractility and the recycling and delivery of integrins to the PM,Citation8 we studied the functions of RHOB and ARF6 in response to motogenic cues. Following HGF stimulation, MET is internalized into the endosomal trafficking network. This observation led us to hypothesize a model whereby a MET-dependent signal activates RHOB in a spatially restricted manner to regulate cytoskeletal dynamics. Accordingly, in response to HGF, MET is rapidly internalized into the endosomal trafficking network and colocalized with RHOB to the endosomes. This was accompanied by enhanced stress fiber formation at the cell leading edge and increase in the number and length of FAs, phenotypes that were lost following RHOB or ARF6 depletion or expression of RHOB lacking the GCI residues.
Cell-matrix adhesions are essential integrators of the cell invasion machinery. In accordance with their roles in FA and actin dynamics, the depletion of ARF6 or RHOB or the expression of RHOBΔGCI enhanced cell dispersion and invasion through a 3D-matrix. Our findings support a model whereby the depletion of ARF6 promotes lysosomal-dependent degradation of RHOB, leading to enhanced FA turnover, reorganization of the actin cytoskeleton, and cell invasion (). Taken together, our observations provide a model through which a MET-dependent signal could activate RHOB in a spatially restricted manner and revealed that the GCI tripeptide sequence of RHOB is required for efficient cytoskeletal rearrangements and sustained invasive properties in response to HGF.
Figure 1. Mechanisms though which ARF6-RHOB regulates breast cancer cell invasion. (a) ARF6 interacts with RHOB. The interaction requires the tripeptide “GCI” (glycine, cysteine, and, isoleucine) residues (188–190) of RHOB and the effector domain regions, Switch I and Switch II (28–73) of ARF6. ARF6 is essential for RHOB protein stability and the regulation of its subcellular localization. Specific targeting of ARF6 to the plasma membrane or endosomal vesicle promotes the recruitment and colocalization of RHOB to these membrane microdomains, thereby controlling cell adhesion by increasing focal adhesion (FA) formation. (b) ARF6 depletion promotes the loss of RHOB from endosomal vesicles and plasma membrane, leading to RHOB degradation. Inhibition of ARF6-RHOB complexes reduces stress fiber formation, increases FA dynamics, promoting breast cancer cell invasion.
Endocytic recycling has emerged as a critical mechanism coordinating F-actin dynamics and directing cell movement downstream of mitogenic growth factors and their receptors, such as MET, by controlling signal intensity and duration, as well as localizing signaling complexes.Citation5,Citation10 While the role of ARF6 in regulating cell migration is complex and likely context-dependent,Citation4 our study demonstrates that, in the context of MET RTK activation, ARF6 plays an important role in the regulation of RHOB stability and subcellular localization, restraining migratory responses. This is consistent with other studies showing that RHOB acts mainly as a suppressor of cell migration and is often lost or downregulated during tumor progression.Citation8 Collectively, our findings suggest a model through which a MET-dependent signal could activate RHOB in a spatially restricted manner and identify a novel mechanism by which ARF6 controls RHOB localization and stability. This indicates a strict requirement for ARF6 and the GCI residues of RHOB for the endosomal and PM targeting of RHOB, consequently regulating membrane trafficking and cancer cell invasion.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Schmid SL. Reciprocal regulation of signaling and endocytosis: implications for the evolving cancer cell. J Cell Biol. 2017;216(9):1–3. doi:10.1083/jcb.201705017.
- Gherardi E, Birchmeier W, Birchmeier C, Woude GV. Targeting MET in cancer: rationale and progress. Nat Rev Cancer. 2012;12(2):89–103. doi:10.1038/nrc3205.
- Lai AZ, Abella JV, Park M. Crosstalk in Met receptor oncogenesis. Trends Cell Biol. 2009;19(10):542–551. doi:10.1016/j.tcb.2009.07.002.
- D’Souza-Schorey C, Chavrier P. ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol. 2006;7(5):347–358. doi:10.1038/nrm1910.
- Parachoniak CA, Luo Y, Abella J, Keen J, Park M. GGA3 functions as a switch to promote Met receptor recycling, essential for sustained ERK and cell migration. Dev Cell. 2011;20(6):751–763. doi:10.1016/j.devcel.2011.05.007.
- Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borist G, Parson T, Horwitz. Cell migration: integrating signals from front to back. Science. 2003;302(5651):1704–1709. doi:10.1126/science.1092053.
- Adamson P, Paterson HF, Hall A. Intracellular localization of the P21rho proteins. J Cell Biol. 1992;119(3):617–627. doi:10.1083/jcb.119.3.617.
- Wheeler AP, Ridley AJ. Why three Rho proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res. 2004;301(1):43–49. doi:10.1016/j.yexcr.2004.08.012.
- Perez-Sala D, Boya P, Ramos I, Herrera M, Stamatakis K. The C-terminal sequence of RhoB directs protein degradation through an endo-lysosomal pathway. PLoS One. 2009;4(12):e8117. doi:10.1371/journal.pone.0008117.
- Zaoui K, Rajadurai CV, Duhamel S, Park M. Arf6 regulates RhoB subcellular localization to control cancer cell invasion. J Cell Biol. 2019;218(11):3812–3826. doi:10.1083/jcb.201806111.
