ABSTRACT
The Notch pathway is an essential signaling system allowing neighboring cells to communicate and accomplish their proper developmental role in physiological condition. Nevertheless, there are many controversies conferring its function in pathological condition, particularly in cancer. It has been discovered that epigenetic regulation, posttranslational modifications, gene overexpression, and mutations may lead to the dysregulation of the Notch pathway. Additionally, Notch-mediated signaling can support tumor-suppressing mechanisms in certain types of cancer or may have oncogenic functions in others. Notch2 is one of the receptors commonly expressed in a variety of cancer cells, including gastric, hematological, and lung cancer. Moreover, it can be dysregulated in other diseases. In efforts to explain the role of Notch2 in the pathogenesis of cancer, recent studies indicated an association between this receptor and dysregulation of miRNAs, tumor-associated stromal cell, and modulation in tumor cells. Consequently, Notch2 function in the carcinogenesis process is unquestionable, whereas information according to the effect of its inhibition in tumor is still obscure. Hence, the aim of our study was to evaluate the current state of knowledge conferring Notch2 inhibition, with a particular focus on its role in cancer.
KEYWORDS:
Introduction
The Notch signaling is evolutionarily conserved pathway and is composed of four receptors (Notch1, Notch2, Notch3, and Notch4) and five canonical ligands (Dll1, Dll2, Dll3, Jag1, and Jag2). Whereas canonical Notch ligands are mostly responsible for the Notch signaling activity, there are non-canonical ligands that can activate Notch and possibly contribute to the pleiotropic effects of Notch signaling.Citation1
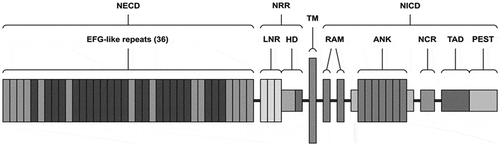
In order to be activated through canonical way, Notch receptors are enzymatically cut by three cleavages. All Notch receptors are produced as a single transmembrane polypeptide in the endoplasmic reticulum and moved to the cell surface through the Golgi apparatus. The structure of Notch2 receptor is presented in . Notch receptors can cooperate with Notch ligands when a ligand of the Delta/Serrate/LAG-2 family (situated on the surface of neighboring cells) binds to the extracellular domain of the Notch receptor and activates proteolytic cleavage by a metalloprotease (a disintegrin and metalloprotease (ADAM)). Furthermore, ADAM10 and/or ADAM17 cleavage produces a substrate for a additional cleavage, generating a Notch extracellular (NECT) form of the receptor, which is a membrane-bound Notch fragment. Following, the presenilin-containing γ-secretase complex, the Notch intracellular domain (NICD) is releasing and corresponds to the activated form of Notch. Moreover, NICD translocases to the nucleus and forms complexes with specific DNA-binding proteins (CBF1/Suppressor of Hairless/LAG-1 and Mastermind/SEL-8) and transcriptionally activates target genesCitation2 such as the Hes (hairy enhance of split) and Hey (hairy/enhancer of split related with YRPW motif) families, c-myc, NF-κB, p21, cyclinD1, and other targets which are not well described yet.Citation3,Citation4 Notch signaling function is known in physiological condition, and it is important for cell to cell communication, control multiple cell differentiation processes during embryonic and adult life.Citation5 Notch2 is described to reveal activity in liver, kidney, ovary, smooth muscle, T and B lymphocyte development. Moreover, according to literature, Notch2 is expressed in hippocampus and cerebellum, and during brain development, it plays a crucial role in the glial cell differentiation.Citation6,Citation7
Figure 1. Structure of the Notch2 receptor. NECD – Notch extracellular domain, NRR – negative regulatory region, TM – transmembrane domain, NICD – Notch intracellular domain, EGF – epidermal growth factor, LNR – cysteine-rich Lin repeats, HD – heterodimerization domain, RAM – RAM domain, ANK – ankyrin repeats, NCR – cysteine response region, TAD – transactivation domain, PEST – transactivation domain rich in proline (P), glutamine (E), serine (S), and threonine (T) residues.
Nevertheless, there are many controversies conferring Notch2 role in a pathological condition, particularly in cancer. According to available literature, several mechanisms are known through which Notch signaling is regulated in cancer. Therefore, it has been discovered that epigenetic regulation, posttranslational modifications, gene overexpression, and mutations may lead to the dysregulation of the Notch pathway.Citation7
Additionally, Notch-mediated signaling can support tumor-suppressing mechanisms in certain types of cancer or may have oncogenic functions in others.Citation7-Citation9 In attempts to explain the role of Notch2 in the pathogenesis of cancer, recent studies indicated an association between this receptor and dysregulation of miRNAs, tumor-associated stromal cell, and modulation in tumor cells.Citation7 Moreover, it has been presented that Notch2 can be downregulated in renal or human non-small cell lung cancer when compared to non-cancerous lung tissuesCitation10 or upregulated in gastric cancer, brain tumors, or lymphocyte malignancies.Citation11-Citation15 Although the role of elevated Notch2 in cancer has been described, the mechanisms of upregulation remain different depending on the type of cancer.
Therefore, in gastric cancer different non-coding RNAs have been found to be linked to the regulation of Notch2. According to the authors, miRNA-23b could be involved in the translation of Notch2.Citation11 Moreover, the scientists gave another option for the regulatory mechanism of Notch2 through miRNA-133a which as a result could inactivate form intracellular form of Notch2 and consequently block pro-oncogenic Notch signaling.Citation12
Other types of malignancies with Notch2 overexpression are brain tumors, such as pilocytic astrocytoma (PA), glioblastoma (GBM), medulloblastoma (MB), and choroid plexus tumors.Citation7 The mechanism leading to Notch2 overexpression in MB occurs with the participation of Jagged1 ligand. According to the results, the majority of MB tumors (35/47 in one study) exhibit Jagged1 ligand overexpression, leading to increasing Notch2 activation and signaling. Furthermore, this action enables MB cell survival, so deregulated Jagged1 expression can decrease this survival via the downregulation of the Notch2 target Hes1.Citation13
Another described in the literature type of cancer, where Notch2 overexpression has been detected is laryngeal squamous cell carcinoma (LSCC) where higher Notch2 expression levels have been detected, and inhibiting Notch2 slowed their growth what has been associated with p-ERK, c-Myc, and Bcl-2 downregulation, as well as Bax upregulation.Citation14
Moreover, it has been found that Notch2 was overexpressed in B cell malignancies, but the underlying pathogenic mechanisms differ according to whether or not the Notch2 gene was mutated in these forms of cancer.Citation15 Consequently, Notch2 function in the carcinogenesis process is undeniable, although there is not much information on the significance of this receptor in cancer development, as well as on the clinical effects of its inhibition in cancer cells. Hence, the aim of the presented study was to evaluate the current state of knowledge conferring Notch2 inhibition, with a particular focus on its role in cancer.
Overview of notch2 inhibitors
Gliotoxin
Gliotoxin is a mycotoxin produced by several species of fungi with a variety of functions. It has been observed that gliotoxin is capable to induce immunosuppressive reactions, influence apoptosis in certain cells of the immune system, and possess anti-inflammatory activity.Citation16
Available information according to gliotoxin as Notch2 inhibitor revealed that targeting canonical Notch2 signaling by this drug is associated with the induction of apoptosis in melanoma, hepatocellular carcinoma (HCC), and pancreas cancer, whereas the other well-known Notch inhibitors are not effective. The potential clinical significance of this discovery was demonstrated in a study with melanoma xenograft mouse model, showing that gliotoxin significantly reduced the tumor volume. Therefore, in the initial stage tumor model, application of gliotoxin revealed a substantial decrease of the mean tumor volume (control group versus treatment group A: 115 mm3 vs. 83 mm3; P = .008) with the greatest tumor mass reduction after 2 weeks (68%). Moreover, in the late-stage tumor model indicated a significant decrease of the mean tumor volume (control group versus treatment group B: 576 mm3 vs. 218 mm3, P = .005) with the maximum tumor mass reduction after 1 week (77%).Citation17 As mentioned above, Notch receptor–ligand interactions activate cleavage of the Notch receptor and release of its intracellular domain from the membrane. NICD localizes to the nucleus where it forms a transcriptionally active complex with the DNA-binding protein CSL and the coactivator Mastermind (MAM) to up-regulate transcription from Notch target genes.Citation18 Therefore, the researchers additionally tested HCC38 and MDA-MB-468 breast cancer cells for their Notch activity and sensitivity to gliotoxin. The researchers examined the Notch2/CSL complex formation process. As a result, HCC38 cells were found to be positive for Notch2/CSL complex formation, whereas in MDA-MB-468 development of this complex was not detected. Furthermore, both cell lines were treated with gliotoxin to evaluate its effect on the above complex formation. Consequently, in HCC38 cells, gliotoxin inhibited Notch2/CSL complex formation and induced apoptosis in a dose-dependent manner; however, MDA-MB-468 cells were resistant to gliotoxin treatment.Citation17
Other research groups indicated that gliotoxin is a potent Notch2 inhibitor and efficiently induces apoptosis in chronic lymphocytic leukemia (CLL) cells. Moreover, it has been discovered, that gliotoxin selectively targets CLL lymphocytes within a protective lymphoid microenvironment. Thus, gliotoxin may potentially target CLL cells in lymphoid organs, which according to the authors, would help in the extermination of CLL. Additional advantage of gliotoxin is that it is effective in a wide range of CLL cases independent of their response or resistance to gamma-secretase inhibitor, which is a common drug used in Notch inhibition.Citation19 Further hypothesis applies the modulatory effect of gliotoxin on Notch signaling redox reaction. Keeping in mind that Notch signaling is sensitive to the above parameter; it is possible to speculate that gliotoxin may directly interfere with the Notch2 transcription factor complex on DNA. This hypothesis is strengthened by the fact that gliotoxin inhibits the formation of DNA‐bound Notch2 complexes.Citation20
Tarextumab (OMP-59R5)
Tarextumab is an anti-Notch2 and anti-Notch3 antibody which selectively binds these receptors. Tarextumab has been confirmed to inhibit tumor growth in patient-derived xenograft tumors. A phase 1b study of tarextumab in combination with other drugs, like etoposide and platinum (EP) in untreated small cell lung cancer (SCLC) patients showed that this combination was well tolerated with dose-dependent anti-tumor activity.Citation21 According to Yen WC at al. targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell existence. The researchers found that anti-Notch2/3, either as a single agent or in combination with other chemotherapeutic agents were effective in epithelial cells derived tumors, including breast, lung, ovarian, and pancreatic cancers. The antitumor effect of anti-Notch2/3 in combination with gemcitabine and nab-paclitaxel was more significant than the combination effect with gemcitabine alone. Other findings indicated that inhibition of both human and mouse Notch2 and Notch3 function and its antitumor activity were characterized by a dual mechanism of action in both tumor and stromal/vascular cells in xenograft experiments. In tumor cells, anti-Notch2/3 inhibited expression of Notch target genes and reduced tumor-initiating cell frequency.Citation22 Conferring further studies on tarextumab in patients with solid tumors, authors paid attention to the dose-limiting toxicity. According to researchers, the toxicity profile of tarextumab is similar to that seen with gamma-secretase inhibitor (GSI). Nevertheless, GSI dosage is limited by the development of secretory diarrhea presumably due to the effects of Notch pathway inhibition on progenitor cells within the intestinal crypts.Citation23,Citation24 The authors remind that the above findings were similar to the results obtained from other preclinical studies which have shown that the immature progenitor cells in the intestine were under the control of Notch signaling and during Notch inhibition, these cells differentiated into secretory goblet cells.Citation25,Citation26 In summary, tarextumab was well tolerated in patients with advanced solid tumors at doses of 2.5 mg/kg weekly, and 7.5 mg/kg every 14 or 21 days, with biomarker evidence of Notch pathway inhibition at these doses.Citation27
Minar1
The major intrinsically disordered Notch2-associated receptor (Minar) was identified as a novel ligand for Notch2 receptor. Ho RX et al. reveal that Minar is located on the cell surface and is highly expressed in endothelial cells in human vascular system. According to the authors, the association between Minar and Notch2 increases its order and stability, and also reduces the degradation of Minar. Moreover, the researchers established that Minar regulates Notch2 activation. Nevertheless, the main function discovered by researchers seems to be inhibition of angiogenesis through Minar–Notch2-dependent manner. However, there are no further experiments to proof this thesis.Citation28
N-acetylcysteine (NAC)
N-acetylcysteine (NAC) is a precursor of intracellular glutathione (GSH) with antioxidant function. Due to its function, NAC has been involved in the prevention and therapy of some types of cancer. Latest experiments conducted by Deng L, et.al provided that Notch2 has been established to be a prognostic marker in Glioblastomas multiforme (GBM), the most aggressive type of cancer that begins within the brain. Moreover, presented data showed that NAC could decrease the protein level of Notch2. Meanwhile, the researchers revealed reduction of the mRNA and protein levels of its downstream targets Hes1 and Hey1. The mechanism of NAC-mediated Notch2 reduction was described by assisting Notch2 degradation through Itch-dependent lysosome pathway. Additionally, the authors indicated that NAC could prevent proliferation, migration, and invasion and might induce apoptosis in GBM cells via targeting Notch2.Citation29
Long non-coding RNA (lncRNA)
Long non-coding RNAs (lncRNAs) belong to a group of non-coding RNAs with more than 200 nucleotides. Non-coding RNAs (ncRNAs), accounting for 98% of the human genome, are classified into several types in view of the length, which include long non-coding RNAs (lncRNAs), microRNAs (miRNAs), circular RNA (circRNAs), and other types. They are the key regulators in many human diseases, together with cancers. Lately, several researchers have concentrated on miRNA regulation via Notch2. There are studies indicated that different types of miRNA could be involved in cell proliferation, migration, and invasion by targeting Notch2 in bladder cancer, gallbladder, non-small cell lung cancer.Citation30-Citation32 Moreover, there are studies indicating that specific LncRNA MIR22HG could inhibit the development of endometrial carcinoma, lung cancer, and hepatocellular carcinoma. The authors obtained that MIR22HG negatively regulated Notch2 signaling. Silencing MIR22HG elevated one of the target genes of Notch-HEY1 and nucleus Notch2 expression. Silencing of Notch2 inhibited cells proliferation, migration, and invasion. Consequently, LncRNA MIR22HG repressed gastric cancer progression through reducing Notch2 signaling.Citation33,Citation34
Conclusions
The importance of the Notch signaling pathway in cancer has been documented already. Moreover, it has been known that Notch can have either oncogenic or tumor-suppressive functions. The mechanisms interfering with Notch2 activity are based on distracting its formation and degradation. Available data according to Notch2 show that it can be deregulated in cancer at mRNA or protein level and maybe a key regulator in cancerogenesis. Moreover, according to literature disrupting the interactions between stromal cells and Notch2 is also an important approach to remove the micro-environmental signals that support tumor growth and survival.Citation9 Therefore, the overview of novel molecules that affect the Notch2 could be one of the options for the prevention or treatment of various types of tumor (). However, as mention above, a proper determination of Notch gene expression in cancer cells depends on many factors, including specific control, tumor cell type, as well as growth factors in the microenvironment, it is essential to recognize that Notch signaling act in tissue-specific and in context-dependent manner when analyzing its function in cancer. Consequently, the role of Notch signaling in cancer cannot be basically linked to either upregulation or downregulation of receptors in tumor tissue. Therefore, the development of mechanism-based inhibitors of Notch signaling could be a perfect option in the treatment of cancer and other diseases.
Table 1. The list of notch2 regulators and clinical implications.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
References
- D’Souza B, Meloty-Kapella L, Weinmaster G. Canonical and non-canonical Notch ligands. Curr Top Dev Biol. 2010;92:1–5.
- Bray S, Bernard F. Notch targets and their regulation. Curr Top Dev Biol. 2010;92:253–275.
- Kopan R, Ilagan MXG. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell. 2009;137:216–233. doi:10.1016/j.cell.2009.03.045.
- Fortini ME. Notch signaling: the core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi:10.1016/j.devcel.2009.03.010.
- Kovall RA, Blacklow SC. Mechanistic insights into Notch receptor signaling from structural and biochemical studies. Curr Top Dev Biol. 2010;92:31–71.
- Varadkar P, Kraman M, Despres D, Ma G, Lozier J, McCright B Notch2 is required for the proliferation of cardiac neural crest-derived smooth muscle.
- Xiu MX, Liu YM. The role of oncogenic Notch2 signaling in cancer: a novel therapeutic target. Am J Cancer Res. 2019 May 1;9(5):837–854. 2019. uscle cells. Dev Dyn. 2008;237(4):1144‐1152. doi:10.1002/dvdy.21502.
- Pancewicz-Wojtkiewicz J, Eljaszewicz A, Kowalczuk O, Niklinska W, Charkiewicz R, Kozłowski M, Miasko A, Moniuszko M. Prognostic significance of Notch ligands in patients with non-small cell lung cancer. Oncol Lett. 2017 Jan;13(1):506–510. doi:10.3892/ol.2016.5420.
- Brzozowa-Zasada M, Piecuch A, Michalski M, Segiet O, Kurek J, Harabin-Słowińska M, Wojnicz R. Notch and its oncogenic activity in human malignancies. Eur Surg. 2017;49(5):199–209. doi:10.1007/s10353-017-0491-z.
- Joanna P, Leszek BP. Differential Notch1 and Notch2 expression in non-small cell lung cancer. Progress Health Sci. 2019;1(9):8–12.
- Huang TT, Ping YH, Wang AM, Ke CC, Fang WL, Huang KH, Lee HC, Chi CW, Yeh TS. The reciprocal regulation loop of Notch2 pathway and miR-23b in controlling gastric carcinogenesis. Oncotarget. 2015;6:18012. doi:10.18632/oncotarget.4000.
- Chen XB, Li W, Chu AX. MicroRNA-133a inhibits gastric cancer cells growth, migration, and epithelial-mesenchymal transition process by targeting presenilin 1. J Cell Biochem. 2019;120:470–480. doi:10.1002/jcb.27403.
- Fiaschetti G, Schroeder C, Castelletti D, Arcaro A, Westermann F, Baumgartner M, Shalaby T, Grotzer MA. NOTCH ligands JAG1 and JAG2 as critical pro-survival factors in childhood medulloblastoma. Acta Neuropathologica Commun. 2014;2:39. doi:10.1186/2051-5960-2-39.
- Zou Y, Fang F, Ding YJ, Dai MY, Yi X, Chen C, Tao ZZ, Chen SM. Notch2 signaling contributes to cell growth, anti-apoptosis and metastasis in laryngeal squamous cell carcinoma. Mol Med Rep. 2016;14:3517–3524. doi:10.3892/mmr.2016.5688.
- Kamga PT, Bassi G, Cassaro AC, Stradoni R, Midolo M, Perbellini O, Krampera M. Role of stromal cell-mediated Notch signaling in AML survival and resistance to chemotherapy. Oncotarget. 2016;7:21713–21727. doi:10.18632/oncotarget.7964.
- Scharf DH, Brakhage AA, Mukherjee PK. Gliotoxin–bane or boon? Environ Microbiol. 2016 Apr;18(4):1096–1109. doi:10.1111/1462-2920.13080.
- Hubmann R, Sieghart W, Schnabl S, Araghi M, Hilgarth M, Reiter M, Demirtas D, Valent P, Zielinski C, Jäger U, et al. Gliotoxin targets nuclear NOTCH2 in human solid tumor derived cell lines in vitro and inhibits melanoma growth in xenograft mouse model. Front Pharmacol. 2017 Jul 7;8:319. doi:10.3389/fphar.2017.00319.
- Yuan Z, Friedmann DR, VanderWielen BD, Collins KJ, Kovall RA. Characterization of CSL (CBF-1, Su(H), Lag-1) mutants reveals differences in signaling mediated by Notch1 and Notch2. J Biol Chem. 2012 Oct 12;287(42):34904–34916. doi:10.1074/jbc.M112.403287.
- Hubmann R, Düchler M, Schnabl S, Hilgarth M, Demirtas D, Mitteregger D, Hölbl A, Vanura K, Le T, Look T, et al. NOTCH2 links protein kinase C delta to the expression of CD23 in chronic lymphocytic leukaemia (CLL) cells. Br J Haematol. 2010 Mar;148(6):868–878. doi:10.1111/j.1365-2141.2009.08024.x.
- Hubmann R, Hilgarth M, Schnabl S, Ponath E, Reiter M, Demirtas D, Sieghart W, Valent P, Zielinski C, Jäger U, et al. Gliotoxin is a potent NOTCH2 transactivation inhibitor and efficiently induces apoptosis in chronic lymphocytic leukaemia (CLL) cells. Br J Haematol. 2013 Mar;160(5):618–629. doi:10.1111/bjh.12183.
- Lu H, Jiang Z. Advances in antibody therapeutics targeting small-cell lung cancer. Adv Clin Exp Med. 2018 Sep;27(9):1317–1323. doi:10.17219/acem/70159.
- Yen WC, Fischer MM, Axelrod F, Bond C, Cain J, Cancilla B, Henner WR, Meisner R, Sato A, Shah J, et al. Targeting Notch signaling with a Notch2/Notch3 antagonist (tarextumab) inhibits tumor growth and decreases tumor-initiating cell frequency. Clin Cancer Res. 2015 May 1;21(9):2084–2095. doi:10.1158/1078-0432.CCR-14-2808.
- Krop I, Demuth T, Guthrie T, Wen PY, Mason WP, Chinnaiyan P, Butowski N, Groves MD, Kesari S, Freedman SJ, et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J Clin Oncol. 2012;30:2307–2313. doi:10.1200/JCO.2011.39.1540.
- Messersmith WA, Shapiro GI, Cleary JM, Jimeno A, Dasari A, Huang B, Shaik MN, Cesari R, Zheng X, Reynolds JM, et al. A phase I, dose-finding study in patients with advanced solid malignancies of the oral gamma-secretase inhibitor PF-03084014. Clin Cancer Res. 2015;21:60–67. doi:10.1158/1078-0432.CCR-14-0607.
- Fre S, Huyghe M, Mourikis P, Robine S, Louvard D, Artavanis-Tsakonas S. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi:10.1038/nature03589.
- van Es JH, van Gijn ME, Riccio O, van den Born M, Vooijs M, Begthel H, Cozijnsen M, Robine S, Winton DJ, Radtke F, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi:10.1038/nature03659.
- Smith DC, Chugh R, Patnaik A, Papadopoulos KP, Wang M, Kapoun AM, Xu L, Dupont J, Stagg RJ, Tolcher, et al. A phase 1 dose escalation and expansion study of Tarextumab (OMP-59R5) in patients with solid tumors. A Invest New Drugs. 2019 Aug;37(4):722–730. doi:10.1007/s10637-018-0714-6.
- Ho RX, Meyer RD, Chandler KB, Ersoy E, Park M, Bondzie PA, Rahimi N, Xu H, Costello CE, Rahimi N. MINAR1 is a Notch2-binding protein that inhibits angiogenesis and breast cancer growth. J Mol Cell Biol. 2018 Jun 1;10(3):195–204. doi:10.1093/jmcb/mjy002.
- Deng J, Liu A-D, Hou G-Q, Zhang X, Ren K, Chen X-Z, Li SSC, Wu Y-S, Cao X. N-acetylcysteine decreases malignant characteristics of glioblastoma cells by inhibiting Notch2 signaling. J Exp Clin Cancer Res. 2019;38:2. doi:10.1186/s13046-018-1016-8.
- Wu X, Chen B, Shi H, Zhou J, Zhou F, Cao J, Sun X. MiR-758-3p suppresses human bladder cancer cell proliferation, migration and invasion by targeting NOTCH2. Exp Ther Med. 2019 May;17(5):4273–4278. doi:10.3892/etm.2019.7400.
- Hua CB, Song SB, Ma HL, Li XZ. MiR-1-5p is down-regulated in gallbladder carcinoma and suppresses cell proliferation, migration and invasion by targeting Notch2. Pathol Res Pract. 2019 Jan;215(1):200–208. doi:10.1016/j.prp.2018.10.013.
- Wang X, Meng Q, Qiao W, Ma R, Ju W, Hu J, Lu H, Cui J, Jin Z, Zhao Y, et al. miR-181b/Notch2 overcome chemoresistance by regulating cancer stem cell-like properties in NSCLC. Stem Cell Res Ther. 2018 Nov 23;9(1):327. doi:10.1186/s13287-018-1072-1.
- Zhang X, Xie K, Zhou H, Wu Y, Li C, Liu Y, Liu Z, Xu Q, Liu S, Xiao D, et al. Role of non-coding RNAs and RNA modifiers in cancer therapy resistance. Mol Cancer. 2020 Mar 2;19(1):47. doi:10.1186/s12943-020-01171-z.
- Li H, Wang Y. Long noncoding RNA (lncRNA) MIR22HG suppresses gastric cancer progression through attenuating NOTCH2 signaling. Med Sci Monit. 2019 Jan 23;25:656–665. doi:10.12659/MSM.912813.
