ABSTRACT
Different intrinsic and extrinsic stress pathways including endoplasmic reticulum (ER) stress converge on the phosphorylation of eukaryotic translation initiation factor 2A (EIF2A, best known as eIF2α), which characterizes the so-called “integrated stress response”. This phosphorylation event is important for the induction of autophagy in response to multiple distinct stressors, as well as for the exposure of calreticulin (CALR) as an “eat me” signal on the surface of the plasma membrane of stressed cells. Both autophagy and CALR exposure are required for immunogenic cell death, a modality of cellular demise that ignites anticancer and antiviral immune responses. In several different cancer types, eIF2α phosphorylation indicates favorable prognosis, correlating with an enhanced antitumor immune response.
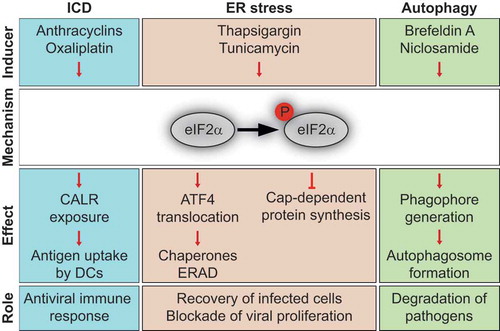
In response to various kinds of stress, eukaryotic translation initiation factor 2A (EIF2A, best known as eIF2α) kinases including eukaryotic translation initiation factor 2 alpha kinase 1 (EIF2AK1, also called HRI for heme-regulated inhibitor) upon heme deficiency or arsenic treatment, eukaryotic translation initiation factor 2 alpha kinase 2 (EIF2AK2, also named PKR for protein kinase R) in the context of viral infection, eukaryotic translation initiation factor 2 alpha kinase 3 (EIF2AK3, best known as PERK for protein PKR-like endoplasmic reticulum kinase) in the framework of endoplasmic reticulum (ER) stress and eukaryotic translation initiation factor 2 alpha kinase 4 (EIF2AK4, also termed GCN2 for general control nonderepressible 2) upon amino acid deprivation are activated and phosphorylate eIF2α. This evolutionary conserved phenomenon is part of the “integrated stress response” which leads to an inhibition of the global (cap-dependent) protein translation while it activates the (cap-independent) synthesis of certain proteins, such as activating transcription factor 4 (ATF4). This results in a shift of the transcriptional program to enhance the production of chaperones that participate in protein folding, as well as in the synthesis of components of the ER-associated degradation (ERAD) machinery for the elimination of misfolded/unfolded proteins. Altogether, these adaptive mechanisms facilitate cellular recovery from transient stress, yet ultimately induce apoptosis when stress is chronic or damage is irreversible.Citation1
As a result, eIF2α phosphorylation is involved in a large panel of intracellular stress pathways, including immunogenic cell stress that can be elicited by viral infection, by treatment with some chemotherapeutics like anthracyclines and oxaliplatin, as well as by physical cues such as photodynamic therapies. In general, inducers of immunogenic cell death (ICD) trigger eIF2α phosphorylation without any activation of the other arms of ER stress.Citation2 This eIF2α-centered stress response promotes the translocation of calreticulin (CALR) from the ER to the plasma membrane, where it acts as an “eat-me” signal for dendritic cells (DCs) through binding to LDL receptor related protein 1 (LRP1).Citation3 The CALR-dependent uptake of dead-cell antigens by DCs is necessary for the initiation of a T lymphocyte response against viral or tumor-associated antigens. In the course of ICD, the release of high-mobility group box 1 (HMGB1) and annexin A1 (ANXA1) into the extracellular space are also required for DC recruitment and activation, through binding to Toll-like receptor 4 (TLR4) and formyl peptide receptor-1 (FPR1), respectively.Citation3
Immunogenic chemotherapies also trigger additional intracellular stress responses such as the inhibition of RNA and protein synthesis, that may be functionally coupled to eIF2α phosphorylation,Citation4 and autophagy, a lysosomal degradation pathway leading to the digestion and recycling of portions of the cytoplasm. In the context of ICD, autophagy facilitates the lysosomal secretion of ATP, which acts on purinergic receptors P2Y2 (P2RY2) and P2X7 (P2RX7) to attract DCs into the tumor bed. Of note, in some instances, eIF2α phosphorylation is required for the induction of autophagy. In yeast, it was shown decades ago that eIF2α phosphorylation is necessary for autophagy induction upon amino acid deprivation. We recently studied the link between these two stress pathways in mammalian cells responding to a large panel of distinct pharmacological autophagy modulators. The induction of autophagic puncta was impaired in response to several but not all autophagy inducers in cells bearing a non-phosphorylable eIF2α mutant or lacking eIF2α kinases.Citation5 Thus eIF2α phosphorylation appears to be central for the manifestation of two hallmarks of ICD, CALR exposure and autophagy, underlining the notion that this phenomenon is pathognomonic for ICD.
High CALR expression is associated with better response to chemotherapeutic ICD inducers in patients with acute myeloid lymphoma (AML), lung or ovarian cancers,Citation6,Citation7 confirming the clinical impact of the eIF2α phosphorylation-CALR axis. Interestingly, even independently of the treatment, CALR exposure on the surface of malignant cells was associated with increased tumor infiltration by T cells and improved clinical outcome in patients with non-small cell lung cancer (NSCLC) or ovarian cancer. In these cancers, CALR exposure was also linked to an elevated level of ER stress.Citation8,Citation9 In addition, EIF2AK3-mediated eIF2α phosphorylation was associated with improved clinical outcome in human epidermal growth factor receptor 2 (HER2)+ breast cancer patients, while the density of tumor infiltrating lymphocytes correlated with efficient responses to treatment with the HER2-targeting antibody trastuzumab, suggesting yet another link between eIF2α phosphorylation and anticancer immune responses.Citation10,Citation11 Besides, increasing eIF2α phosphorylation by SAL003 (an inhibitor of eIF2α phosphatase) improved the sensitivity of breast cancers to trastuzumab in preclinical models.Citation10 ONC201, a first-in-class small molecule that antagonizes the dopamine receptor D2, triggers the eIF2α-ATF4-CHOP ER stress pathway while promoting immune cell infiltration in lymphoma patients.Citation12 Altogether, these results confirm the clinical importance of eIF2α phosphorylation as a biomarker that correlates with improved immunosurveillance at baseline, as well as therapeutic responses to a variety of anticancer drugs.
To sum up, eIF2α phosphorylation can be regarded as a central signaling hub () that favors the elimination of tumors and virus-infected cells, because [i] it impairs the replication of intracellular pathogens through the inhibition of protein translation, [ii] it promotes their degradation by autophagy/xenophagy (through the formation of autophagolysosomes within cells) and by phagocytosis and [iii] it facilitates the recognition of infected/stressed cells by DCs, ultimately leading to the activation and proliferation of cytotoxic T-lymphocytes subsequent to the cross-presentation of viral or tumor-associated antigens. It is important to note that many viruses encode proteins that inhibit eIF2α kinases or activate eIF2α phosphatases, thus blocking cell-autonomous antiviral pathways and evading immune recognition.Citation3 It is tempting to speculate, yet remains to be demonstrated that, under the pressure of immunoselection, malignant cells may inactivate eIF2α phosphorylation with a similar outcome, namely the evasion from immunosurveillance.
Figure 1. Role of eIF2α phosphorylation in various stress pathways.
Disclosure of Potential Conflicts of Interest
GK and OK are cofounders of Samsara Therapeutics.
Acknowledgments
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; AMMICa US23/CNRS UMS3655; Association pour la recherche sur le cancer (ARC); Association “Le Cancer du Sein, Parlons-en!”; Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; European Research Area Network on Cardiovascular Diseases (ERA-CVD, MINOTAUR); Gustave Roussy Odyssea, the European Union Horizon 2020 Project Oncobiome; Fondation Carrefour; High-end Foreign Expert Program in China (GDW20171100085), Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx Immuno-Oncology (ANR-18-IDEX-0001); the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); and the SIRIC Cancer Research and Personalized Medicine (CARPEM). J.H. was supported by the foundation Philanthropia. L.B. is supported by BMS.
References
- Hetz C, Papa FR. The unfolded protein response and cell fate control. Mol Cell. 2018;69(2):1–3. doi:10.1016/j.molcel.2017.06.017.
- Bezu L, Sauvat A, Humeau J, Gomes-da-Silva LC, Iribarren K, Forveille S, Garcia P, Zhao L, Liu P, Zitvogel L, et al. eIF2alpha phosphorylation is pathognomonic for immunogenic cell death. Cell Death Differ. 2018;25(8):1375–1393. doi:10.1038/s41418-017-0044-9.
- Galluzzi L, Vitale I, Warren S, Adjemian S, Agostinis P, Martinez AB, Chan TA, Coukos G, Demaria S, Deutsch E, et al. Consensus guidelines for the definition, detection and interpretation of immunogenic cell death. J Immunother Cancer. 2020;8(1):e000337. doi:10.1136/jitc-2019-000337.
- Humeau J, Sauvat A, Cerrato G, Xie W, Loos F, Iannantuoni F, Bezu L, Lévesque S, Paillet J, Pol J, et al. Inhibition of transcription by dactinomycin reveals a new characteristic of immunogenic cell stress. EMBO Mol Med. 2020;12(5):e11622. doi:10.15252/emmm.201911622.
- Humeau J, Leduc M, Cerrato G, Loos F, Kepp O, Kroemer G, et al. Phosphorylation of eukaryotic initiation factor-2α (eIF2α) in autophagy. Cell Death Dis. 2020. doi:10.1038/s41419-020-2642-6.
- Garg AD, Elsen S, Krysko DV, Vandenabeele P, de Witte P, Agostinis P. Resistance to anticancer vaccination effect is controlled by a cancer cell-autonomous phenotype that disrupts immunogenic phagocytic removal. Oncotarget. 2015;6(29):26841–26860. doi:10.18632/oncotarget.4754.
- Aurelius J, Möllgård L, Kiffin R, Ewald Sander F, Nilsson S, Thorén FB, Hellstrand K, Martner A. Anthracycline-based consolidation may determine outcome of post-consolidation immunotherapy in AML. Leuk Lymphoma. 2019;60(11):2771–2778. doi:10.1080/10428194.2019.1599110.
- Fucikova J, Becht E, Iribarren K, Goc J, Remark R, Damotte D, Alifano M, Devi P, Biton J, Germain C. Calreticulin expression in human non-small cell lung cancers correlates with increased accumulation of antitumor immune cells and favorable prognosis. Cancer Res. 2016;76(7):1746–1756. doi:10.1158/0008-5472.CAN-15-1142.
- Kasikova L, Hensler M, Truxova I, Skapa P, Laco J, Belicova L, Praznovec I, Vosahlikova S, Halaska MJ, Brtnicky T, et al. Calreticulin exposure correlates with robust adaptive antitumor immunity and favorable prognosis in ovarian carcinoma patients. J Immunother Cancer. 2019;7(1):312. doi:10.1186/s40425-019-0781-z.
- Darini C, Ghaddar N, Chabot C, Assaker G, Sabri S, Wang S, Krishnamoorthy J, Buchanan M, Aguilar-Mahecha A, Abdulkarim B, et al. An integrated stress response via PKR suppresses HER2+ cancers and improves trastuzumab therapy. Nat Commun. 2019;10(1):2139. doi:10.1038/s41467-019-10138-8.
- Ignatiadis M, Van den Eynden G, Roberto S, Fornili M, Bareche Y, Desmedt C, Rothé F, Maetens M, Venet D, Holgado E, et al. Tumor-infiltrating lymphocytes in patients receiving trastuzumab/pertuzumab-based chemotherapy: A TRYPHAENA substudy. J Natl Cancer Inst. 2019;111(1):69–77. doi:10.1093/jnci/djy076.
- Romaguera JE, Lee HJ, Tarapore R, Prabhu V, Allen J, Schalop L, Zloza A, Ok CY, Sadimin ET, Schenkel J, et al. Integrated stress response and immune cell infiltration in an ibrutinib-refractory mantle cell lymphoma patient following ONC 201 treatment. Br J Haematol. 2019;185(1):133–136. doi:10.1111/bjh.15271.
