ABSTRACT
Developmental pathways play an important role in cancer. We have recently demonstrated that the constitutive activation of the developmental transcription factor SOX6 via the fusion oncoprotein EWSR1-FLI1 (Ewing sarcoma breakpoint region 1 – Friend leukemia virus integration 1) contributes to the aggressive phenotype of Ewing sarcoma but on another hand provides an opportunity for targeted therapy.
Ewing sarcoma was first described as ‘diffuse endothelioma of bone’ by James Ewing in 1921.Citation1 Back then, a link toward a bone-related disease was already established. Although the origin of Ewing sarcoma is still debated, accumulating evidence suggests mesenchymal stem cells or early committed osteo-chondrogenic progenitor cells as possible cells of origin.Citation2
Indeed, Ewing sarcoma most commonly arises in bone such as long bones and pelvis and mainly affects adolescents with an age peak of 15 y (i.e. still growing individuals).Citation2 Especially during endochondral ossification, the developmental transcription factor SOX6 (SRY-Box Transcription factor 6) is transiently highly expressed and plays an important role in the proliferation and differentiation of bone cells.Citation3 Intriguingly, SOX6 is also highly expressed in Ewing sarcoma cells that display a highly proliferative but undifferentiated phenotype. Genetically, Ewing sarcoma cells harbor pathognomonic EWSR1-ETS (Ewing sarcoma breakpoint region 1 – E26 specific) fusion oncogenes (in most cases EWSR1-FLI1; Ewing sarcoma breakpoint region 1 – Friend leukemia virus integration 1), which encode aberrant transcription factors.Citation2 Accordingly, we investigated the potential contribution of SOX6 in Ewing sarcoma tumorigenesis, and whether EWSR1-FLI1 takes part in the regulation of SOX6 expression.
In our study, we revealed that EWSR1-FLI1 is able to hijack SOX6, which is then constitutively, but variably expressed in Ewing sarcoma cells and primary tumors.Citation4 Furthermore, we discovered that this constitutive overexpression of SOX6 contributes – at least in part – to the aggressive and proliferative phenotype observed in Ewing sarcoma, but also constitutes a therapeutic vulnerability toward the oxidative stress-inducing drug Elesclomol.Citation4
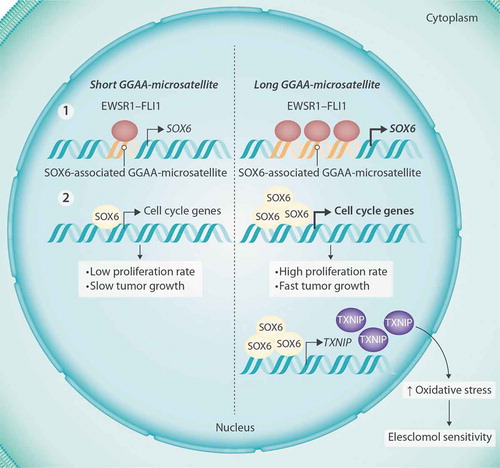
It is known that the fusion oncoprotein EWSR1-FLI1 is able to bind to GGAA-microsatellites (mSats), thereby converting them into enhancer-like regulatory DNA elements. Interestingly, the enhancer-activity of EWSR1-FLI1-bound GGAA-mSats increases with the number of consecutive GGAA-repeats.Citation2 Through this mechanism, Ewing sarcoma cells express a highly specific and unique gene-signature including EGR2, MYBL2, and NROB1.Citation5–Citation7 In our recent study, we found that EWSR1-FLI1 binds to an intronic GGAA-mSat within the SOX6 gene, whose number of GGAA-repeats correlated positively with its enhancer activity and cellular SOX6 expression levels. The variability at this SOX6-associated GGAA-mSat may explain the high degree of inter-tumor heterogeneity of SOX6 expression across Ewing sarcoma tumors.Citation4
To better understand the role of SOX6 in Ewing sarcoma tumorigenesis and progression, we performed transcriptomic analyses, which revealed a loss of proliferation-related gene-signatures upon SOX6-silencing. These observations were validated in functional in vitro and in vivo assays that demonstrated a contribution of SOX6 to proliferation, spheroidal growth, and tumorigenesis of Ewing sarcoma cells.Citation4
Next, we were wondering whether the constitutively high SOX6 expression could constitute a specific vulnerability that could be exploited therapeutically. To this end, we screened for drugs in published databasesCitation4 that were able to inhibit Ewing sarcoma cell growth at physiologically tolerable doses and that were in particular active in Ewing sarcoma cells with high SOX6 expression levels. Unexpectedly, this screen identified the oxidative-stress inducer Elesclomol among the top hits.Citation4 Elesclomol is a small-molecule that increases intracellular oxidative stress beyond a certain threshold ultimately triggering apoptosis.Citation8
Drug-response assays in SOX6-high versus SOX6-low expressing Ewing sarcoma cells showed that SOX6 functionally confers Elesclomol sensitivity. Further experiments unraveled the mechanism behind this observation:Citation4 We demonstrated that SOX6 stimulates the expression of TXNIP (Thioredoxin Interacting Protein) – an inhibitor of the thioredoxin antioxidant system.Citation9 Indirect and direct suppression of TXNIP as well as rescue experiments showed that SOX6-induced TXNIP expression contributes to elevated intracellular and mitochondrial levels of oxidative stress, which in turn makes Ewing sarcoma cells very sensitive toward Elesclomol.Citation4
It has already been shown that Elesclomol in combination with doxorubicin can inhibit cell growth in breast cancer cells.Citation10 Since doxorubicin is one of the main drugs applied in current Ewing sarcoma treatment protocols,Citation2 it is tempting to speculate that SOX6 could be used as predictive biomarker for Elesclomol-treatment alone or in combination with other oxidative stress-inducing drugs such as doxorubicin for Ewing sarcoma patients with high intratumoral SOX6 expression levels.
In summary, our experiments showed how the aberrant activation of a developmental transcription factor (here SOX6) through a dominant oncogene (here EWSR1-FLI1) can promote malignancy but also provide opportunities for target therapeutic interventionCitation4 (). Yet, there are still open questions: For instance, the precise mechanism through which SOX6 induces TXNIP expression in Ewing sarcoma needs to be further elucidated. Also, since SOX6 usually require cell-type-specific co-factors for transcriptional regulation and because SOX6 acts in other cancer types as a tumor suppressor, it is of interest to identify its specific cofactors being operative in Ewing sarcoma cells. These and other questions will be addressed in future experiments, and as such our study represents just the end of the beginning in our understanding of the role(s) of SOX6, TXNIP, and oxidative stress in this devastating disease.
Figure 1. Dual role of SOX6 in Ewing sarcoma progression and sensitivity toward Elesclomol. Variability of a regulatory GGAA-microsatellite located in intron 1 of SOX6 modulates EWSR1-FLI1 binding and SOX6 expression levels in Ewing sarcoma cells. High SOX6 expression on the one hand promotes tumor growth, but on the other hand creates a therapeutic vulnerability toward Elesclomol via upregulation of intracellular oxidative stress. EWSR1-FLI1, Ewing sarcoma breakpoint region 1 – Friend leukemia virus integration 1; SOX6, SRY-Box Transcription factor 6; TXNIP, Thioredoxin Interacting Protein.
Disclosure of potential conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
We thank sommersault1828 for the professional illustration.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Ewing J. Diffuse endothelioma of bone. Proc NY Pathol Soc. 1921;12:1–3.
- Grünewald TGP, Cidre-Aranaz F, Surdez D, Tomazou EM, de Álava E, Kovar H, Sorensen PH, Delattre O, Dirksen U. Ewing sarcoma. Nat Rev Dis Primers. 2018;4:5. doi:10.1038/s41572-018-0003-x.
- Smits P, Dy P, Mitra S, Lefebvre V. Sox5 and Sox6 are needed to develop and maintain source, columnar, and hypertrophic chondrocytes in the cartilage growth plate. J Cell Biol. 2004;164:747–758. doi:10.1083/jcb.200312045.
- Marchetto A, Ohmura S, Orth MF, Knott MML, Colombo MV, Arrigoni C, Bardinet V, Saucier D, Wehweck FS, Li J. Oncogenic hijacking of a developmental transcription factor evokes vulnerability toward oxidative stress in Ewing sarcoma. Nat Commun. 2020;11:2423. doi:10.1038/s41467-020-16244-2.
- Grünewald TG, Bernard V, Gilardi-Hebenstreit P, Raynal V, Surdez D, Aynaud -M-M, Mirabeau O, Cidre-Aranaz F, Tirode F, Zaidi S, et al. Chimeric EWSR1-FLI1 regulates the Ewing sarcoma susceptibility gene EGR2 via a GGAA microsatellite. Nat Genet. 2015;47:1073–1078. doi:10.1038/ng.3363.
- Monument MJ, Johnson KM, McIlvaine E, Abegglen L, Watkins WS, Jorde LB, Womer RB, Beeler N, Monovich L, Lawlor ER, et al. Clinical and biochemical function of polymorphic NR0B1 GGAA-microsatellites in Ewing sarcoma: a report from the Children’s Oncology Group. PLoS One. 2014;9:e104378. doi:10.1371/journal.pone.0104378.
- Musa J, Cidre-Aranaz F, Aynaud -M-M, Orth MF, Knott MML, Mirabeau O, Mazor G, Varon M, Hölting TLB, Grossetête S, et al. Cooperation of cancer drivers with regulatory germline variants shapes clinical outcomes. Nat Commun. 2019;10:4128. doi:10.1038/s41467-019-12071-2.
- Kirshner JR, He S, Balasubramanyam V, Kepros J, Yang C-Y, Zhang M, Du Z, Barsoum J, Bertin J. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol Cancer Ther. 2008;7:2319–2327. doi:10.1158/1535-7163.MCT-08-0298.
- Zhou J, Chng W-J. Roles of thioredoxin binding protein (TXNIP) in oxidative stress, apoptosis and cancer. Mitochondrion. 2013;13:163–169. doi:10.1016/j.mito.2012.06.004.
- Qu Y, Wang J, Sim M-S, Liu B, Giuliano A, Barsoum J, Cui X. Elesclomol, counteracted by Akt survival signaling, enhances the apoptotic effect of chemotherapy drugs in breast cancer cells. Breast Cancer Res Treat. 2010;121:311–321. doi:10.1007/s10549-009-0470-6.
