ABSTRACT
Colorectal cancer (CRC) is one of the most important malignancies and causes of cancer-related deaths worldwide. Cancer stem cell markers identification could be helpful to acquire important prognostic information and develop new treatment regimens. This study aimed to evaluate the expression of OCT4 and NANOG in CRC patients and their clinical significance.
Totally 359 CRC samples were stained for OCT4 and NANOG expression using tissue microarray. The correlation between their expression and clinical and pathological features was explored.
The majority of CRC cases showed low-level expression of OCT4 (80%) and NANOG (75%). Lower expression of OCT4 was more often detected in CRC cases with no vascular involvement (P = .01). Also, a trend found between low level of OCT4 expression and absence of distant metastasis or lymph node involvement (P = .07 and P = .09, respectively). Surprisingly, a significant positive correlation was observed between NANOG expression and cellular differentiation (P = .05). Our combined analysis demonstrated that OCT4 low/NANOG low phenotype has frequently seen in colorectal cancer cases with no vascular invasion (P = .05).
Our observations indicated that higher expression of OCT4 and NANOG can confer malignant and aggressive behavior to CRC. Evaluation of the co-expression of these cancer stem cell markers can serve a new diagnostic and prognostic approach in CRC patients. These findings also suggested that simultaneous expression of OCT4 and NANOG can be considered as a therapeutic marker for targeted therapy of CRC, especially in advanced stages.
Introduction
Colorectal cancer (CRC) represents the second cause of cancer-related deaths and the third most prevalent malignancy worldwide.Citation1,Citation2 The global burden of CRC is showing an alarming trend, with a predicted upraise in newly diagnosed cases from 2 × 10 6 in 2018 to 2.2 × 10 6 in 2030 .Citation3 Although advances in cancer treatment have improved the outcomes in CRC patients, a considerable proportion of cases (40%) experienced recurrence .Citation4 Thus, novel therapeutic approaches are needed to implement the existing armamentarium of available treatment options. A large body of evidence supports the hypothesis that cancer development is driven by a fraction of the cancer cells population called cancer stem cells (CSCs) or tumor-initiating cells (TICs) .Citation5,Citation6 They possess some unique characteristics, including self-renewal, tumor initiation and maintenance attitude, multi-lineages differentiation capacity, and hyper-malignant phenotype. Some of these features have been associated with CSCs’ chemotherapy and radiotherapy resistance, occurrence of metastasis, as well as early tumor relapse .Citation7,Citation8
Octamer-binding transcription factor 4 (OCT4), also known as POU domain, class 5, transcription factor 1 (POU5F1), is implicated in cell pluripotency and induction of embryonic stem cell-like state in fibroblasts or stem cells. OCT4 can be found in pre-gastrulation embryos, developing endoderm, and neuroectoderm pluripotent stem cells. As part of the POU (Pit-Oct-Unc) transcription factors family, it appears to regulate cell growth and differentiation. Evaluation of the OCT4 expression has been documented in large varieties of malignant tumors .Citation9,Citation10 More interestingly, the critical role of OCT4 in chemo/radioresistance of CSCs has been investigated .Citation11
NANOG is a transcription factor that plays an important role in both self-renewal and pluripotency of embryonic stem cells .Citation12–14 Additionally, this molecule exerts its effects through the regulation of cell proliferation, tumorigenicity, invasive behavior, and resistance to therapies. Dysregulation of NANOG has been reported in various tumors, such as breast and oral squamous cell carcinoma (OSCC) .Citation15–17 The functions of NANOG are dependent on OCT4 expression and co-expression of NANOG and OCT4 has been shown to be associated with pancreatic cancer and OSCC .Citation18 The objective of this study was to investigate the expression of NANOG and OCT4 in CRC patients and their potential tumor markers.
Materials and methods
Patient selection and sample preparation
In this cross-sectional study, 359 formalin-fixed paraffin-embedded (FFPE) CRC samples were retrieved from the archives of three referral Tehran hospitals, including Rasoul Akram, Firoozgar, and Hasheminejad Hospitals from 2009 to 2014. These tissues were taken before any treatment. Clinical and pathological variables collected from patients’ medical records included: pathologic tumor stage (pTNM staging), tumor volume, vascular invasion, perineural invasion, as well as regional lymph node involvement. The perineural invasion was mentioned as perineural invasion and intraneural invasion .Citation19 In this study, the perineural invasion was evaluated by expert pathologists using hematoxylin and eosin slides and documented in the pathological analysis reports. Interestingly, the perineural invasion is correlated with more aggressive behavior as well as poor prognosis in some malignancies, including pancreas, and prostate cancers .Citation20 The patient information was obtained anonymously to preserve confidentiality.
Tissue microarray construction
Colorectal TMA blocks were prepared from FFPE CRC samples as previously described .Citation21 For this purpose, representative areas were selected on hematoxylin and eosin slides. Chosen regions in donor blocks were punched and 0.6-mm diameter samples cylinders arrayed into a new recipient paraffin block by Tissue Arrayer, MiniCore® (ALPHELYS, Plaisir, France). Colorectal TMA blocks were constructed in three copies and adjacent normal CRC tissue was included in each block to evaluate OCT4 and NANOG staining patterns.
Immunohistochemistry staining
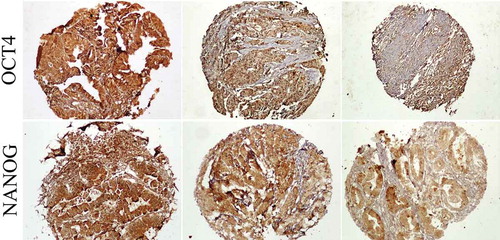
Expression patterns of potential CSC markers OCT4 and NANOG were measured as previously described using immunohistochemical staining .Citation22,Citation23 The TMA sections were deparaffinized with xylene and dehydrated using serially diluted alcohol. The endogenous peroxidase activity inhibited by incubating the slides in 3% hydrogen peroxide in methanol for 20 minutes. After washing antigen retrieval was done by immersing the slides in citrate buffer (pH = 6.0) for OCT4 and Tris-EDTA buffer (pH = 9.0) for NANOG for 10 minutes in an autoclave. Next, incubation at 4°C with primary antibodies (OCT4: rabbit polyclonal anti-OCT4, ab18976, Abcam, Cambridge, UK, and NANOG: rabbit monoclonal anti-NANOG, ab109250, Abcam Cambridge, UK) was performed overnight. Color development was carried out using diaminobenzidine (DAB) solution and then counterstained with hematoxylin. Seminoma was used as a positive control in each immunohistochemical staining for both OCT4 and NANOG antibodies. Replacement of primary antibodies with a washing buffer was used as a negative control.
Evaluation of immunostaining scores
The stained slides were evaluated by two expert pathologists (M. Barodabi & M. Panahei) using a multi-headed microscope in a semi-quantitative manner. At first, the stained slides were examined at 10 × magnification to achieve a general overview of expression distribution, then the stained cores were examined for localization at higher magnification. Staining positivity was graded as 0 (no staining), 1 (fade), 2 (moderate), and 3 (strong). In addition, the percentage of positive tumor cells was scored as positive tumor cells < 25%, between 25 to 50%, between 50 to 75%, and more than 75%. The final score, called Histochemical score (H-score), was calculated with the formula staining positivity × percentage of positive tumor cells, ranging from 0 to 300 .Citation24,Citation25
Statistical analysis
Statistical analyses were performed using SPSS for Windows, version 16. Descriptive data were presented as mean ± standard deviation (SD) or number and percentage. Independent t-test and one-way ANOVA or their non-parametric counterparts (Mann–Whitney U test and Kruskal–Wallis H test) were used for the evaluation of the statistical association between H-scores and the clinical-pathologic characteristics of the patients. A Chi-squared test was used for the evaluation of the statistical association between OCT4/NANOG status and the clinical-pathologic characteristics of the patients. Pearson’s or spearman’s correlation coefficient tests were used for the evaluation of potential correlations between the level of expressions and clinical-pathological factors. A P value of ≤ 0.05 was considered as statistically significant.
Results
Patients’ characteristics
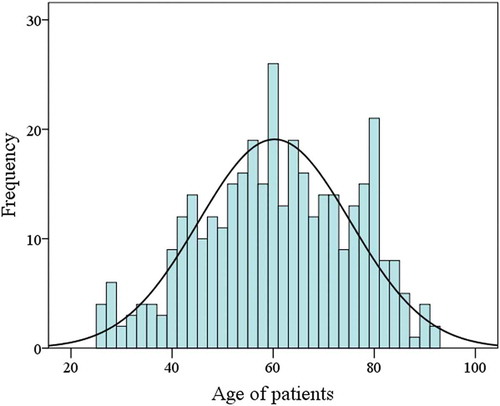
In this cross-sectional study, 359 colorectal cancer patients were included with a mean age of 60 ± 15 and approximately equal male to female ratio. Distribution analysis of included colorectal cancer patients based on their age using histogram plot is shown in . One hundred twenty-eight (36%) and 206 (58%) tumors showed good and moderate differentiation, whereas 20 (6%) colorectal samples had poor differentiation. The presence of metastasis was found in 20 (6%) cases versus 331 (94%). Furthermore, the neural invasion was seen in 64 (18%) CRC patients. All clinical-pathological information is summarized in .
Table 1. Correlations between OCT4 and NANOG expressions with clinicopathological parameters in colorectal Cancer
Expression patterns of OCT4 in colorectal cancer samples and its correlation with clinical and pathologic characteristics
Expression patterns of OCT4 in colorectal cancer samples were grouped as low (≤ mean of H-sore) and high (> mean of H-score) where the cutoff = 7 (). Low OCT4 expression levels were observed in 288 (80%) samples, while 71 (20%) showed high expression levels of this marker. Our analysis indicated that lower expression of OCT4 was more often detected in colorectal cancer cases with no vascular involvement (P = .01). More interestingly, a trend was found between low OCT4 expression and the absence of metastasis (P = .07) or lymph node involvement (P = .09). There were no statistically significant differences between OCT4 expression and other clinical or pathological characteristics ().
Expression patterns of NANOG in colorectal cancer samples and correlation with clinical and pathologic characteristics
NANOG immunostaining patterns were subdivided into low (≤ mean of H-sore) and high (> mean of H-sore) where the cutoff = 24 (). The majority of colorectal cancer specimens showed low expression of NANOG (267, 75%), while high expression of this antigen was seen in 92 (25%) stained cores. Evaluation of the correlation between NANOG expression and clinical and pathological factors was explored. There was a significant inverse correlation between NANOG expression and sigmoid colon localization of the tumor (P = .04). Additionally, a positive significant correlation was observed between NANOG expression and tumor differentiation (expressed in terms of H-score, P = .05). There was no significant correlation between NANOG immunoreactivity and other pathological parameters ().
Association between OCT4 expression, NANOG expression and the patients’ clinical and pathological characteristics
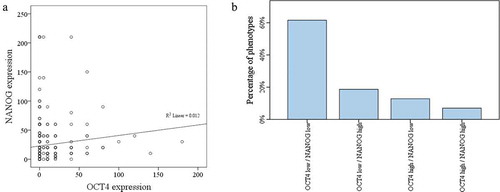
The correlation between OCT4 and NANOG is shown in . Immunohistochemical expression of OCT4 and NANOG showed a positive significant correlation (P = .03). Four different phenotypes were defined; low expression of both markers (OCT4 low/NANOG low), low expression of one marker (OCT4 high/NANOG low or OCT4 low/NANOG high) and high expression of both markers (OCT4 high/NANOG high) (). Our analysis demonstrated that OCT4 low/NANOG low phenotype was more often detected in colorectal cancer cases with no vascular invasion (P = .05). No significant correlation was seen between these phenotypes and pathological factors.
Discussion
Surgery with or without adjuvant chemotherapy and irradiation depending on cancer stage and tumor location represents the conventional treatment for colorectal cancer, but a high percentage of patients experience drug resistance, relapse, or recurrence as well as distant metastasis .Citation26 Colorectal cancer stem cell subpopulations have been hypothesized to be responsible for tumor growth under stress, pharmacological resistance, disease progression, and metastasis. Chemotherapy and radiation induce stemness genes in cancer cells, leading to CSCs enrichment in tumor tissue. In an effort to fully eradicate the disease, it is crucial to find the molecular targets that are uniquely displayed by CSCs, as to not target normal stem cells .Citation27
Accumulating evidence indicates that transcription factors OCT4 and NANOG are involved as key mediators in self-renewal, and pluripotency of CSCs, tumorigenesis and drug resistance. The expression of these biological markers is associated with worse prognosis in different types of malignant tumors .Citation28,Citation29 In the present study, we investigated the expression of OCT4 and NANOG and their potential as a tumor marker in colorectal cancer patients. A considerable percentage (80%) of patients showed low expression levels of OCT4. Lower expression of OCT4 in turn showed a positive correlation with the absence of vascular invasion. In addition, a trend was seen between the low expression of OCT4 and the absence of metastasis or lymph node involvement. However, different studies indicated that OCT4 expression in tumor tissues is enhanced in different cancers .Citation9,Citation30 In the study conducted by Talebi et al., OCT4 expression was analyzed in normal and colon adenocarcinoma tissues. Their results showed no statistically significant association between OCT4 expression and cancer incidence .Citation31 Meanwhile, Saigusa et al. measured OCT4 expression in patients with rectal cancer undergoing chemoradiotherapy .Citation32 They found an elevation of OCT4 and its association with distant recurrence and poor prognosis. It has been previously hypothesized that OCT4 is not expressed in normal conditions in adult cells. Matsuoka et al showed expression of CSC markers Oct3/4 in 44% of 290 gastric cancers and its correlation with prognosis .Citation33 Amini et al also demonstrated that elevated expression of OCT4 in younger patients with colorectal cancer is associated with advanced disease stages and poor outcomes .Citation34 It has been shown that metastasis is a complicated process that is initiated by the conversion of epithelial cells to mesenchymal cells which is known as epithelial-mesenchymal transition (EMT) .Citation35 One of the critical factors in this process is OCT4 and higher expression of this marker in tumors which progress shows that this CSC is associated with aggressive behavior .Citation36
We also examined the immunohistochemical expression of NANOG in the same set of colorectal cancers. Our analysis demonstrated that 75% of samples had low NANOG expression levels. Surprisingly, a significant positive correlation was detected between NANOG expression and differentiation as well as tumor preferential localization to the sigmoid colon. Evaluation of the role of NANOG as a potential biomarker in colorectal cancer reported heterogeneous results. Fischedick et al. concluded that NANOG is not an oncogenic factor, whereas Vaz et al. could not find any association between NANOG levels and survival rate in their study conducted on patients with stage II colon cancer .Citation37,Citation38 On the other hand, Meng et al. analyzed 175 colorectal cancer patients and found an association between NANOG expression levels and tumor stage and metastasis to the lymph nodes, hypothesizing a prognostic role for NANOG .Citation39 Yasuda et al. found that NANOG expression is increased in ulcerative colitis, whilst decreased in colorectal cancer associated with ulcerative colitis. They also showed the same results for OCT4 .Citation40 Xu et al demonstrated measurable levels of NANOG mRNA and protein in colorectal cancer samples with higher histological grade, advanced stage, the involvement of lymph node and liver metastases, Citation41 while Saiki et al reported no significant association between mRNA expression of NANOG with clinic-pathological characteristics of colorectal cancer specimens .Citation42 In a recent meta-analysis, NANOG was introduced as a valuable and independent prognostic factor in patients with gastrointestinal luminal cancers .Citation43
We also found a positive significant correlation between OCT4 and NANOG. The OCT4 low/NANOG low phenotype was found in more than sixty percent of colorectal cancer cases. Interestingly, this phenotype was frequently seen in colorectal cancers with no vascular invasion. Ibrahim and his colleagues showed that expression of NANOG is regulated by OCT4/SOX2 complex and the expression levels of other pluripotency genes are regulated by three transcription factors; OCT4/SOX2 and NANOG, therefore analysis of these axes have a critical role in diagnostic, prognostic as well as follow up treatment in colorectal cancer patients. Developing therapeutic approaches based on the unique properties and functions of colorectal CSCs markers has the potential to improve survival and clinical outcomes. A combination of conventional chemo/radiotherapy regimens and CSC targeted therapies could represent a good option in colorectal cancer patients’ management .Citation44–46
Conclusions
Taken together, our findings demonstrated that higher expressions of OCT4 and NANOG can confer malignant and aggressive behavior to colorectal cancer. Additionally, co-expression of OCT4 and NANOG can represent a novel diagnostic and therapeutic approaches in colorectal cancer; however further studies are required to investigate the mechanisms that are implicated by this co‐expression.
Ethics approval
The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We acknowledge the grant by Deputy for Research and Technology, Ministry of Health and Medical Education, IR Iran (Grant no: 700/169).
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019 Jan;69(1):1–6. doi:10.3322/caac.21551.
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018 Nov;68(6):394–424.
- Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691. doi:10.1136/gutjnl-2015-310912.
- Ciombor KK, Wu C, Goldberg RM. Recent therapeutic advances in the treatment of colorectal cancer. Annu Rev Med. 2015;66(1):83–95. doi:10.1146/annurev-med-051513-102539.
- Bajaj J, Diaz E, Reya T. Stem cells in cancer initiation and progression. J Cell Biol. 2020 Jan 6;219(1). doi:10.1083/jcb.201911053.
- Yadav AK, Desai NS. Cancer stem cells: acquisition, characteristics, therapeutic implications, targeting strategies and future prospects. Stem Cell Rev Reports. 2019 Jun 15;15(3):331–355. doi:10.1007/s12015-019-09887-2.
- Atashzar MR, Baharlou R, Karami J, Abdollahi H, Rezaei R, Pourramezan F, Zoljalali Moghaddam SH. Cancer stem cells: A review from origin to therapeutic implications. J Cell Physiol. 2020 Feb;235(2):790–803. doi:10.1002/jcp.29044.
- Wong AL, Bellot GL, Hirpara JL, Pervaiz S. Understanding the cancer stem cell phenotype: A step forward in the therapeutic management of cancer. Biochem Pharmacol. 2019 Apr 1;162:79–88.
- Vijayakumar G, Narwal A, Kamboj M, Sen R. Association of SOX2, OCT4 and WNT5A expression in oral epithelial dysplasia and oral squamous cell carcinoma: an immunohistochemical study. Head Neck Pathol. 2020 Jan;4:1–9.
- Panayiotou T, Michael S, Zaravinos A, Demirag E, Achilleos C, Strati K. Human papillomavirus E7 binds Oct4 and regulates its activity in HPV-associated cervical cancers. PLoS Pathog. 2020 Apr 16;16(4):e1008468. doi:10.1371/journal.ppat.1008468.
- Mohiuddin IS, Wei SJ, Kang MH. Role of OCT4 in cancer stem-like cells and chemotherapy resistance. Biochimica Biophysica Acta Mol Basis Dis. 2020 Apr 1;1866(4):165432.
- Jeter CR, Yang T, Wang J, Chao HP, Tang DG. Concise review: NANOG in cancer stem cells and tumor development: an update and outstanding questions. Stem Cells. 2015;33(8):2381–2390. doi:10.1002/stem.2007.
- Gong S, Li Q, Jeter CR, Fan Q, Tang DG, Liu B. Regulation of NANOG in cancer cells. Mol Carcinog. 2015;54(9):679–687. doi:10.1002/mc.22340.
- Mahalaxmi I, Devi SM, Kaavya J, Arul S, Balachandar V, Santhy KS. New insight into NANOG: A novel therapeutic target for ovarian cancer (OC). Eur J Pharmacol. 2019 Jun 5;852:51–57.
- Dehghan Harati M, Rodemann HP, Toulany M. Nanog signaling mediates radioresistance in ALDH-positive breast cancer cells. Int J Mol Sci. 2019 Jan;20(5):1151. doi:10.3390/ijms20051151.
- de Vicente JC, Rodríguez-Santamarta T, Rodrigo JP, Allonca E, Vallina A, Singhania A. Donate-Pérez del Molino P, García-Pedrero JM. The Emerging Role of NANOG as an Early Cancer Risk Biomarker in Patients with Oral Potentially Malignant Disorders. J Clin Med. 2019 Sep;8(9):1376.
- Ye T, Li J, Sun Z, Liu Y, Kong L, Zhou S, Tang J, Wang J, Xing HR. Nr5a2 promotes cancer stem cell properties and tumorigenesis in nonsmall cell lung cancer by regulating Nanog. Cancer Med. 2019 Mar;8(3):1232–1245. doi:10.1002/cam4.1992.
- Grubelnik G, Boštjančič E, Pavlič A, Kos M, Zidar N. NANOG expression in human development and cancerogenesis. Exp Biol Med. 2020 Mar;245(5):456–464. doi:10.1177/1535370220905560.
- Cao Y, Deng S, Yan L, Gu J, Li J, Wu K, Cai K. Perineural invasion is associated with poor prognosis of colorectal cancer: a retrospective cohort study. Int J Colorectal Dis. 2020 Mar;16:1–9.
- Yang Y, Huang X, Sun J, Gao P, Song Y, Chen X, Zhao J, Wang Z. Prognostic value of perineural invasion in colorectal cancer: a meta-analysis. J Gastrointestinal Surg. 2015 Jun 1;19(6):1113–1122. doi:10.1007/s11605-015-2761-z.
- Sadeghi A, Roudi R, Mirzaei A, Zare Mirzaei A, Madjd Z, Abolhasani M. CD44 epithelial isoform inversely associates with invasive characteristics of colorectal cancer. Biomark Med. 2019;13(6):419–426. doi:10.2217/bmm-2018-0337.
- Korourian A, Roudi R, Shariftabrizi A, Kalantari E, Sotoodeh K, Madjd Z. Differential role of Wnt signaling and base excision repair pathways in gastric adenocarcinoma aggressiveness. Clin Exp Med. 2017;17(4):505–517. doi:10.1007/s10238-016-0443-0.
- Kalantari E, Asadi Lari MH, Roudi R, Korourian A, Madjd Z. Lgr5High/DCLK1High phenotype is more common in early stage and intestinal subtypes of gastric carcinomas. Cancer Biomarkers. 2017;20(4):563–573. doi:10.3233/CBM-170383.
- Roudi R, Korourian A, Shariftabrizi A, Madjd Z. Differential expression of cancer stem cell markers ALDH1 and CD133 in various lung cancer subtypes. Cancer Invest. 2015;33(7):294–302. doi:10.3109/07357907.2015.1034869.
- Mansoori M, Roudi R, Abbasi A, Abolhasani M, Abdi Rad I, Shariftabrizi A, Madjd Z. High GD2 expression defines breast cancer cells with enhanced invasiveness. Exp Mol Pathol. 2019;109:25–35. doi:10.1016/j.yexmp.2019.05.001.
- Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, Watanabe T. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065. doi:10.1038/nrdp.2015.65.
- Hirata A, Hatano Y, Niwa M, Hara A, Tomita H. Heterogeneity of colon cancer stem cells. Adv Exp Med Biol. 2019;1139:115–126.
- Zhang J, Espinoza LA, Kinders RJ, Lawrence SM, Pfister TD, Zhou M, Veenstra TD, Thorgeirsson SS, Jessup JM. NANOG modulates stemness in human colorectal cancer. Oncogene. 2013;32(37):4397–4405. doi:10.1038/onc.2012.461.
- Gazouli M, Roubelakis MG, Theodoropoulos GE, Papailiou J, Vaiopoulou A, Pappa KI, Nikiteas N, Anagnou NP. OCT4 spliced variant OCT4B1 is expressed in human colorectal cancer. Mol Carcinog. 2012;51(2):165–173. doi:10.1002/mc.20773.
- Tiwari D, Ray Das C, Sultana R, Kakoti S, Aasif Khan M, Dongre A, Husain SA, Bose PD, Bose S. Impact of modulation of telomerase and cancer stem-cell marker OCT4 axis in cervical cancer pathogenesis with underlying HPV16 infection. 2020 Apr;121(4):2782–279.
- Talebi A, Kianersi K, Beiraghdar M. Comparison of gene expression of SOX2 and OCT4 in normal tissue, polyps, and colon adenocarcinoma using immunohistochemical staining. Adv Biomed Res. 2015;4:234.
- Saigusa S, Tanaka K, Toiyama Y, Yokoe T, Okugawa Y, Ioue Y, Miki C, Kusunoki M. Correlation of CD133, OCT4, and SOX2 in rectal cancer and their association with distant recurrence after chemoradiotherapy. Ann Surg Oncol. 2009 Dec 1;16(12):3488–3498. doi:10.1245/s10434-009-0617-z.
- Matsuoka J, Yashiro M, Sakurai K, Kubo N, Tanaka H, Muguruma K, Sawada T, Ohira M, Hirakawa K. Role of the stemness factors sox2, oct3/4, and nanog in gastric carcinoma. J Sur Res. 2012;174(1):130–135. doi:10.1016/j.jss.2010.11.903.
- Amini AQ, Samo KA, Memon AS. Colorectal cancer in younger population: our experience. J Pak Med Assoc. 2013;63:1275–1277.
- Trivanovic D, Krstic J, Jaukovic A, Bugarski D, Santibanez JF. Mesenchymal stromal cell engagement in cancer cell epithelial to mesenchymal transition. Developmen Dynam. 2018;247(3):359–367.
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol. 2019;20(2):69–84. doi:10.1038/s41580-018-0080-4.
- Fischedick G, Wu G, Adachi K, Arauzo-Bravo MJ, Greber B, Radstaak M, Köhler G, Tapia N, Iacone R, Anastassiadis K, et al. Nanog induces hyperplasia without initiating tumors. Stem Cell Res. 2014;13(2):300–315. doi:10.1016/j.scr.2014.08.001.
- Vaz MA, Martinez JC, Devesa JM, Trill JD, Abraira V, Riquelme A, Carrato A. Prognostic value of stem cell quantification in stage II colon cancer. PLoS One. 2014;9(2):e88480. doi:10.1371/journal.pone.0088480.
- Meng HM, Zheng P, Wang XY, Liu C, Sui HM, Wu SJ, Zhou J, Ding Y-Q, Li J. Over-expression of Nanog predicts tumor progression and poor prognosis in colorectal cancer. Cancer Biol Ther. 2010;9(4):295–302. doi:10.4161/cbt.9.4.10666.
- Yasuda H, Tanaka K, Okita Y, Araki T, Saigusa S, Toiyama Y, Yokoe T, Yoshiyama S, Kawamoto A, Inoue Y, et al. CD133, OCT4, and NANOG in ulcerative colitis-associated colorectal cancer. Oncol Lett. 2011;2(6):1065–1071. doi:10.3892/ol.2011.415.
- Xu F, Dai C, Zhang R, Zhao Y, Peng S, Jia C. Nanog: a potential biomarker for liver metastasis of colorectal cancer. Dig Dis Sci. 2012;57(9):2340–2346. doi:10.1007/s10620-012-2182-8.
- Saiki Y, Ishimaru S, Mimori K, Takatsuno Y, Nagahara M, Ishii H, Yamada K, Mori M. Comprehensive analysis of the clinical significance of inducing pluripotent stemness-related gene expression in colorectal cancer cells. Ann Surg Oncol. 2009;16(9):2638–2644. doi:10.1245/s10434-009-0567-5.
- Liang C, Zhao T, Ge H, Xu Y, Ren S, Yue C, Li G, Wu J. The clinicopathological and prognostic value of Nanog in human gastrointestinal luminal cancer: A meta-analysis. Int J Surg (London, England). 2018;53:193–200. doi:10.1016/j.ijsu.2018.03.050.
- Akbarzadeh KM, Safary A, Barar J, Ajoolabady A, Somi MH, Omidi Y. Multifunctional nanomedicines for targeting epidermal growth factor receptor in colorectal cancer. Cellular Mol Life Sci. 2020 Mar;77(6):997–1019.
- Khiavi MA, Safary A, Somi MH. Recent advances in targeted therapy of colorectal cancer: impacts of monoclonal antibodies nanoconjugates. BioImpacts. 2019;9(3):123. doi:10.15171/bi.2019.16.
- Safary A, Moniri R, Hamzeh-Mivehroud M, Dastmalchi S. Highly efficient novel recombinant L-asparaginase with no glutaminase activity from a new halo-thermotolerant Bacillus strain. BioImpacts. 2019;9(1):15. doi:10.15171/bi.2019.03.