ABSTRACT
In search of anti-aging interventions with differential effects on normal and cancer cells, we show that cycles of a fasting-mimicking diet plus pharmacological doses of vitamin C can be effective in targeting KRAS-mutant cancers. This approach represents a promising strategy able to protect the organism while killing cancer cells.
Periodic fasting or fasting-mimicking diet (FMD) cycles have been shown to reduce metabolic markers/risk factors associated with aging and age-related diseases such as diabetes, cardiovascular diseases and cancer in both mice and humans.Citation1 Clinical application of fasting or FMDs in oncology is also emerging with several clinical trials completed or ongoing.Citation2,Citation3
FMDs are caloric-restricted plant–based diets containing low proteins, low sugar and high fats which represent a more feasible and safer option to water-only fasting.Citation1 Importantly, our recent studies showed that FMDs delay tumor progression and potentiate chemotherapy efficacy through both immune-independent and immune-dependent mechanisms, while protecting healthy tissues from chemo-associated side effects in different cancer models.Citation4,Citation5 These phenomena are known as differential stress sensitization and differential stress resistance, respectively.Citation4,Citation5
In the recent years, vitamin C at pharmacological doses has reemerged as a potential anti-cancer molecule.Citation6,Citation7 In the presence of metals, and particularly iron, high dose of vitamin C exerts a pro-oxidant action by generating hydrogen peroxide and hydroxyl radicals via Fenton chemistry.Citation7 Recent findings have shown that tumor cells bearing activating mutation in Kirsten Rat Sarcoma viral oncogene homologue, best known as KRAS, are more susceptible to pharmacological dose of vitamin C than KRAS-wild type ones.Citation6 KRAS mutations have been detected in the most lethal tumor types (in ~45% of colorectal cancers, ~90% of pancreatic ductal adenocarcinomas and in ~30% of lung adenocarcinomas), and they are frequently associated to resistance to the majority of standard treatments.Citation8 So far, attempts to selectively target RAS signaling have shown limited efficacy in the clinic, pointing to the need for new treatments effective against these aggressive tumors.
To address this need, in our recent work,Citation9 we show that FMD cycles potentiate high-dose vitamin C anti-cancer effects in a range of cancer types. In particular, we provide evidence that in vitro an FMD-like culture condition, referred to as Short-Term Starvation (STS), synergizes with vitamin C in selectively killing KRAS-mutant tumor cells derived from colorectal, pancreatic and lung cancers. Consistent with the differential sensitivity that we observed in cancer cell lines, colorectal cancer cells genetically modified to express the mutant form of KRAS are more vulnerable to STS + vitamin C toxic effects than their KRAS-wild type counterpart.
In mice, cycles of FMD potentiate high-dose vitamin C in delaying tumor progression in xenograft, syngeneic and orthotopic models of colon cancer. Importantly, the FMD and vitamin C combo therapy does not cause toxicity to normal cells in vitro and it is safe and well tolerated in different mouse strains.
Our findings reveal that the selective efficacy of FMD and high-dose vitamin C in cancer cells with a KRAS-mutant background is caused by the differential regulation of the stress-inducible protein Heme Oxygenase-1 (HO-1). HO-1, by catabolizing heme in carbon oxide (CO), biliverdin and free iron, which is rapidly sequestered by HO-1-dependent ferritin induction, exerts anti-apoptotic, anti-inflammatory and anti-oxidant properties. For all these reasons, HO-1 is frequently over-expressed as a defense to chemotherapy in different tumor types and it is associated with resistance against standard treatments.Citation10 We found that KRAS-mutant cancer cells respond to vitamin C treatment by up-regulating HO-1, and consequently limiting vitamin C pro-oxidant action ().
Figure 1. FMD sensitizes KRAS-mutant cancer cells to vitamin C. In nutrient-rich condition (left), vitamin C’s toxicity to cancer cells is mostly blocked by the up-regulation of heme-oxygenase-1 (HO-1), which decreases free reactive iron pool (Fe2+) by inducing ferritin (FTH) expression. Fasting-mimicking diet (FMD) reverts the vitamin C mediated HO-1 up-regulation (right), thus increasing the reactive iron pool (Fe2+) and, together with FMD-induced reactive oxygen species (ROS) production, boosts pro-oxidant reactions and Fenton chemistry causing DNA damage (yellow bolts) and cell death. The FMD effect is reversed by treatment with antioxidants such as glutathione (GSH) and N-acetyl cysteine (NAC), HO-1 activator hemin, iron chelator desferrioxamine (DFO) and H2O2 scavenger catalase.
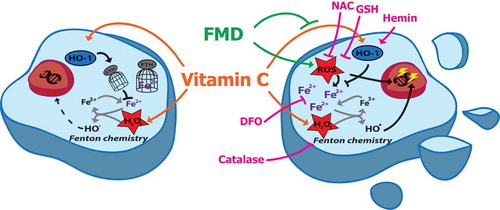
Our data shows that FMD is able to revert HO-1 up-regulation and because of the consequent reduction in ferritin levels, leads to an increase in free reactive iron and oxygen species causing DNA damage and cell death (). Consistent with our findings, antioxidants such as glutathione and N-acetyl cysteine, hydrogen peroxide scavenger catalase, and the iron chelator desferrioxamine revert STS-dependent sensitization to vitamin C by quenching the pro-oxidant reactions and hydrogen peroxide generation.
Interestingly, in KRAS-wild type tumor cells, HO-1 expression is not affected upon vitamin C treatment, and in these wild type cells FMD/STS exerts an opposite effect by up-regulating instead of reducing HO-1 levels.
In agreement with our previous studies demonstrating differential stress sensitization by fasting/FMD in tumor cells and mice, this study points to KRAS mutations as one of the mediators of these differential effects on HO-1/Ferritin expression and death in normal and cancer cells. Consistent with our findings, HO-1 activation suppresses the ability of STS to potentiate vitamin C toxicity, whereas its pharmacological inhibition or genetic down-regulation mimics STS in sensitizing cancer cells to the treatment.
In support of our discovery, the analysis from the Cancer Genome Atlas Database (TCGA) indicates that patients bearing KRAS-mutant colon adenocarcinoma, but not KRAS-wild type colon tumors, with low intratumor ferritin expression level display a longer 3- and 5-y overall survival compared to patients bearing high intratumor ferritin mRNA levels, thus supporting the role of HO−1/ferritin regulation in the progression of colon cancers harboring KRAS mutation.
Our previous studies showed that FMD cycles potentiate chemotherapy efficacy in different tumor types,Citation4,Citation5 thus we also investigated whether this novel treatment based on the combination of two interventions known to delay cellular aging could also boost the effectiveness of standard treatment. Interestingly, we found that the nontoxic FMD + vitamin C combination therapy is as effective as oxaliplatin + vitamin C in delaying tumor progression while the triple FMD, vitamin C and chemotherapy combination treatment is the most effective.
Together with other ongoing studies, these findings reveal new possibilities for the use of multiple interventions which are nontoxic or may even be beneficial to normal cells and organs in cancer treatment. In particular, FMD and pharmacological vitamin C therapy could represent a novel promising intervention to be tested in future clinical trials for the treatment of KRAS-mutant cancers.
Disclosure of potential conflicts of interest
In accordance with Taylor & Francis policy and ethical obligation as a researcher, V.D.L is reporting that he has equity interest in L-Nutra, a company that may be affected by the research reported in the enclosed paper.
Additional information
Funding
References
- Brandhorst S, Choi IY, Wei M, Cheng C, Sedrakyan S, Navarrete G, Dubeau L, Yap L, Park R, Vinciguerra M, et al. A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22(1):86‐99. doi:10.1016/j.cmet.2015.05.012.
- de Groot S, Vreeswijk MP, Welters MJ, Gravesteijn G, Boei JJ, Jochems A, Houtsma D, Putter H, van der Hoeven JJ, Nortier JW, et al. The effects of short-term fasting on tolerance to (neo) adjuvant chemotherapy in HER2-negative breast cancer patients: a randomized pilot study. BMC Cancer. 2015;15:1. Published 2015 Oct 5. doi:10.1186/s12885-015-1663-5.
- Bauersfeld SP, Kessler CS, Wischnewsky M, Jaensch A, Steckhan N, Stange R, Kunz B, Brückner B, Sehouli J, Michalsen A, et al. The effects of short-term fasting on quality of life and tolerance to chemotherapy in patients with breast and ovarian cancer: a randomized cross-over pilot study. BMC Cancer. 2018;18(1):476. Published 2018 Apr 27. doi:10.1186/s12885-018-4353-2.
- Raffaghello L, Lee C, Safdie FM, Wei M, Madia F, Bianchi G, Longo VD. Starvation-dependent differential stress resistance protects normal but not cancer cells against high-dose chemotherapy. Proc Natl Acad Sci U S A. 2008;105(24):8215‐8220. doi:10.1073/pnas.0708100105.
- Di Biase S, Lee C, Brandhorst S, Manes B, Buono R, Cheng CW, Cacciottolo M, Martin-Montalvo A, de Cabo R, Wei M, et al. Fasting-mimicking diet reduces HO-1 to promote T cell-mediated tumor cytotoxicity. Cancer Cell. 2016;30(1):136146. doi:10.1016/j.ccell.2016.06.005.
- Yun J, Mullarky E, Lu C, Bosch KN, Kavalier A, Rivera K, Roper J, Chio IIC, Giannopoulou EG, Rago C, et al. Vitamin C selectively kills KRAS and BRAF mutant colorectal cancer cells by targeting GAPDH. Science. 2015;350(6266):1391‐1396. doi:10.1126/science.aaa5004.
- Schoenfeld JD, Sibenaller ZA, Mapuskar KA, Wagner BA, Cramer-Morales KL, Furqan M, Sandhu S, Carlisle TL, Smith MC, Abu Hejleh T, et al. O2- and H2O2-mediated disruption of Fe metabolism causes the differential susceptibility of NSCLC and GBM cancer cells to pharmacological ascorbate. Cancer Cell. 2017;32(2):268. doi:10.1016/j.ccell.2017.07.008.
- Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov. 2014;13(11):828‐851. doi:10.1038/nrd4389.
- Di Tano M, Raucci F, Vernieri C, Caffa I, Buono R, Fanti M, Brandhorst S, Curigliano G, Nencioni A, de Braud F, et al. Synergistic effect of fasting-mimicking diet and vitamin C against KRAS mutated cancers. Nat Commun. 2020;11(1):2332. Published 2020 May 11. doi:10.1038/s41467-020-16243-3.
- Jozkowicz A, Was H, Dulak J. Heme oxygenase-1 in tumors: is it a false friend? Antioxid Redox Signal. 2007;9(12):2099–3. doi:10.1089/ars.2007.1659.
