ABSTRACT
Valosin-containing protein (VCP) is essential for proteostasis during many cellular processes. However, it remains uncertain how its diverse functions are selectively regulated. We recently showed that DNA damage-induced Ser784 phosphorylation specifically increases VCP function for the DNA damage response and significantly influences the survival of chemotherapy-treated breast cancer patients.
The evolutionarily conserved DNA damage response (DDR) consists of intimately connected cellular processes which collectively maintain genome stability and determine cell fate. Many DDR events are orchestrated by proteostatic changes influencing protein level and localization in a spatiotemporally choreographed manner. An important aspect of proteostasis during DDR is proteasomal degradation of lysine 48 (K48)-linked polyubiquitinated proteins. This is best understood on chromatin, especially around DNA damage sites, where rapid protein reorganization takes place to enable DNA repair and checkpoint signalingCitation1. Studies in the last decade have demonstrated that efficient degradation of many chromatin-associated proteins depends on VCP (Valosin-Containing Protein), an essential and highly abundant AAA+ (ATPases Associated with diverse cellular Activities) ATPase.Citation2 Functioning as a barrel-shaped homo-hexamer, VCP utilizes energy from ATP hydrolysis within its central ATPase domains to extract and unfold polyubiquitinated proteins from various organelles and cellular structures (e.g., endoplasmic reticulum, endosome, chromatin, ribosome) to facilitate their turnover.Citation3 Most substrates interact with the N-terminal domain of VCP indirectly through ubiquitin-binding cofactors such as the dimeric NPL4 (Nuclear Protein Localization protein 4) and UFD1 (Ubiquitin recognition Factor in ER-associated Degradation 1) complex ().
Figure 1. DNA damage-induced Ser784 phosphorylation selectively increases VCP (valosin-containing protein) activity for chromatin-associated protein degradation. (a) Schematics showing the domain structure of monomeric VCP (left) and 3D structure of a functional VCP hexamer (right). The N-terminal domain of VCP interacts with the majority of ubiquitin-binding cofactors such as NPL4 (Nuclear Protein Localization protein 4) and UFD1 (Ubiquitin recognition Factor in ER-associated Degradation 1). D1 and D2 are the central ATPase domains. Ser784 is located in the structurally disordered C-terminal tail of VCP. (b) Working model depicting the selective increase of nuclear VCP activity by DNA damage-induced Ser784 phosphorylation with regard to chromatin-associated protein degradation. In the absence of DNA damage, unphosphorylated VCP extracts its chromatin-associated poly-ubiquitinated substrates at a normal rate. Under DNA-damaging conditions, Ser784 phosphorylation turns VCP into a more efficient protein segregase presumably to extract more chromatin-associated substrates that are functionally important for DNA damage response.
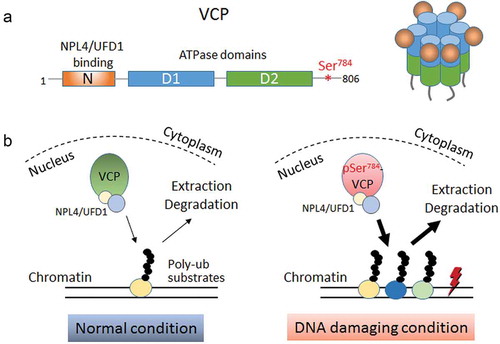
Given the involvement of VCP in diverse proteostatic processes, it is reasonable to speculate that mechanisms may exist to selectively modulate its activity in response to specific cellular stresses. Upon DNA damage, VCP undergoes phosphorylation at Ser784 in its C-terminal tail (). This was first discovered by Livingstone et al. in 2005 using a cross-reacting phospho-CHK2 antibodyCitation4 and subsequently confirmed in 2007 by unbiased proteomic profiling of phosphatidylinositol 3-kinase-related kinases (PIKK) substrates in response to ionizing radiation and ultraviolet treatments.Citation5,Citation6 Ser784 can be phosphorylated by all three master kinases in the PIKK family (Ataxia-Telangiectasia Mutated, ATM; Ataxia Telangiectasia and Rad3-related, ATR; DNA-dependent Protein Kinase, DNA-PK) in response to diverse genotoxic agents and treatments. These observations suggested early on that Ser784 phosphorylation of VCP likely plays an important role in DDR. However, this theory remained untested for 15 years until our recent study.Citation7
Similar to Livingstone et al.Citation4 we serendipitously detected pSer784-VCP in human breast cancer samples using a cross-reacting phospho-PFN1 antibody. Motivated by the chemotherapy-dependent correlation with patient survival, we identified the unknown nuclear antigen of the phospho-PFN1 antibody to be pSer784-VCP. Using a custom-generated pSer784-VCP-specific monoclonal antibody, we confirmed the identity of the DNA damage-induced nuclear antigen of the cross-reacting antibody in cell lines and tissues. Interestingly, Ser784 phosphorylation is a relatively late and lingering DDR event and detected both at DNA damage foci and within soluble nucleoplasm. Subsequent characterization of VCP knockdown and rescue cell lines revealed that Ser784 phosphorylation is important for DNA damage repair and PIKK-dependent checkpoint signaling, and cells expressing the phospho-resistant VCP(S784A) mutant are hypersensitive to a broad range of genotoxic agents including the poly (ADP-ribose) polymerase (PARP) inhibitors. Thus, our data for the first time uncover the functional significance and clinical relevance of Ser784 phosphorylation of VCP.Citation7
On a mechanistic level, our study suggests that the functional importance of Ser784 phosphorylation for DDR is due, at least partially, to the increased VCP activity in chromatin-associated degradation. We found that VCP knockdown causes significant K48-polyubiquitin buildup on chromatin which can be fully rescued by the phospho-mimetic (S784D, serine784 to aspartate) but not phospho-resistant (S784A, serine784 to alanine) VCP mutants. In comparison, the rescuing abilities of these two VCP mutants with regard to the buildup of nucleoplasmic K48-polyubiquitin do not differ significantly.Citation7 These findings are consistent with the well-known importance of VCP for chromatin-associated degradation during DDR and suggest that Ser784 phosphorylation may be a selective functional accelerator in this regard (). However, the potential effects of Ser784 phosphorylation on soluble nuclear proteins cannot be ruled out. Our finding that Ser784 phosphorylation increases the level of HIF1α,Citation7 a soluble nuclear substrate of VCPCitation8 despite its lack of obvious connection to DDR, raises the interesting possibility that Ser784 phosphorylation may differentially affect VCP activity toward protein substrates in a context-dependent fashion. Future research to identify and characterize off-chromatin nuclear substrates of VCP will be necessary to test this theory, and further improve our understanding of the functional involvement of VCP in DDR.
Another interesting finding in our study is that Ser784 phosphorylation, within the C-terminal tail of VCP, decreases its interaction with N-domain-binding cofactors NPL4/UFD1 and K48-polyubiquitins,Citation7 indicative of long-range inter-domain conformational change. Notably, an early report by Klein et al. identified several AKT (AK strain Transforming, also known as protein kinase B) phosphorylation sites in VCP (Ser352, Ser746, and Ser748), and reported that an increase in cellular AKT activity decreases VCP/polyubiquitin interaction.Citation9 Thus, phosphorylation of VCP may be a general mechanism to release substrates in response to different stimuli. In the case of Ser784 phosphorylation, it is tempting to speculate that it triggers the release of chromatin-associated substrates from VCP to speed up their proteasomal degradation and enables faster recycling of VCP for more rounds of protein extraction.
In addition to mechanistic insights, our study also reveals for the first time cancer relevance of pSer784-VCP. By staining three independent tissue microarrays consisting of >3000 breast tumor samples, we observed a significant correlation between high pSer784-VCP levels and poor patient survival in a chemotherapy-dependent fashion, particularly for those in the triple-negative subgroupCitation5. Given that baseline pSer784-VCP (trigged by endogenous DNA damage) in pre-treatment samples was used in our analyses, we most likely have underestimated the true prognostic value of pSer784-VCP which will be reexamined by future experiments using drug-induced pSer784-VCP levels. Collectively, our study has two important therapeutic implications. First, low pSer784-VCP levels may be used as a novel predictive biomarker to select patients who can benefit from genotoxic chemotherapies. Second, tumors containing high levels of pSer784-VCP may benefit from chemo-sensitization by PIKK inhibitors many of which are in clinical development.Citation10
Disclosure of potential conflicts of interest
Provisional patent has been filed for the monoclonal pSer784-VCP antibody as a predictive biomarker for cancer chemotherapies.
Additional information
Funding
References
- Polo SE, Jackson SP. Dynamics of DNA damage response proteins at DNA breaks: a focus on protein modifications. Genes Dev. 2011;25:1–3. doi:10.1101/gad.2021311.
- Vaz B, Halder S, Ramadan K. Role of p97/VCP (Cdc48) in genome stability. Front Genet. 2013;4:60. doi:10.3389/fgene.2013.00060.
- Ye Y, Tang WK, Zhang T, Xia D, Mighty A. “Protein extractor” of the cell: structure and function of the p97/CDC48 ATPase. Front Mol Biosci. 2017;4:39. doi:10.3389/fmolb.2017.00039.
- Livingstone M, Ruan H, Weiner J, Clauser KR, Strack P, Jin S, Williams A, Greulich H, Gardner J, Venere M, et al. Valosin-containing protein phosphorylation at Ser784 in response to DNA damage. Cancer Res. 2005;65:7533–7540. doi:10.1158/0008-5472.CAN-04-3729.
- Matsuoka S, Ballif BA, Smogorzewska A, McDonald ER 3rd, Hurov KE, Luo J, Bakalarski CE, Zhao Z, Solimini N, Lerenthal Y, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi:10.1126/science.1140321.
- Stokes MP, Rush J, Macneill J, Ren JM, Sprott K, Nardone J, Yang V, Beausoleil SA, Gygi SP, Livingstone M, et al. Profiling of UV-induced ATM/ATR signaling pathways. Proc Natl Acad Sci U S A. 2007;104:19855–19860. doi:10.1073/pnas.0707579104.
- Zhu C, Rogers A, Asleh K, Won J, Gao D, Leung S, Li S, Vij KR, Zhu J, Held JM, et al. Phospho-Ser(784)-VCP is required for DNA damage response and is associated with poor prognosis of chemotherapy-treated breast cancer. Cell Rep. 2020;31:107745. doi:10.1016/j.celrep.2020.107745.
- Alexandru G, Graumann J, Smith GT, Kolawa NJ, Fang R, Deshaies RJ. UBXD7 binds multiple ubiquitin ligases and implicates p97 in HIF1alpha turnover. Cell. 2008;134:804–816. doi:10.1016/j.cell.2008.06.048.
- Klein JB, Barati MT, Wu R, Gozal D, Sachleben LR Jr., Kausar H, Trent JO, Gozal E, Rane MJ. Akt-mediated valosin-containing protein 97 phosphorylation regulates its association with ubiquitinated proteins. J Biol Chem. 2005;280:31870–31881. doi:10.1074/jbc.M501802200.
- Brandsma I, Fleuren EDG, Williamson CT, Lord CJ. Directing the use of DDR kinase inhibitors in cancer treatment. Expert Opin Investig Drugs. 2017;26:1341–1355. doi:10.1080/13543784.2017.1389895.
