ABSTRACT
The clinical introduction of magnetic hyperthermia therapy (MHT) has been hindered by current available agents with poor magnetic-to-thermal conversion efficiency and biocompatibility. It is believed that the genetically engineered magnetic nanocages of encapsulin-produced magnetic iron oxide nanocomposites (eMIONs) have great potential as clinically translatable MHT agents for cancer magneto-catalytic theranostics.
As an emerging clinical approach for minimally invasive treatment of cancer since 1957, magnetic hyperthermia therapy (MHT) primarily involves the generation of heat in a local region of the body by using magnetic particles with the application of an externally applied alternating magnetic field (AMF), leading to the achievement of therapeutic effect. MHT has been explored in the treatment of a variety of cancers, such as brain, breast, lung, liver, spine, and so on.Citation1 The effectiveness of MHT mainly depends upon the efficiency of magnetic-to-thermal conversion of the agents. In addition, the safety of the agents must also be ensured prior to the clinical translation of MHT. As for the application in diagnostic and therapeutic medicine, the ideal agents suitable for MHT are required to be of proper chemical composition, physical properties, and biocompatible and can be easily synthesized with low cost.
Magnetic thermal induction agents play an important role in the biomedical applications of MHT by affecting the safe dosage in clinic. With the development of nanotechnology, magnetic nanoparticles (MNPs) have been employed in MHT and become attractive agents owing to the ability of generating heat when exposed to high-frequency magnetic field. Moreover, the synthesis and investigations of MNPs have further driven the progress of MHT for cancer treatment.Citation2 Among the different magnetic thermal induction agents, iron oxide nanoparticles (IONs) have been extensively investigated as MHT agents due to their relatively high biocompatibility and heating capacity. Despite significant research efforts that have been devoted to improve the properties of IONs, certain challenges still exist in conventional IONs in terms of insufficient magnetic-to-thermal conversion efficiency and poor stability when exposed in the AMF, which largely restrict their practical applications.Citation3 Therefore, it is crucial to develop new strategies to improve the conversion efficiency and biocompatibility of IONs for the clinical translation of MHT.
To address these critical problems, the biologically inspired technology, such as biomineralization, has been applied for the development and improvement of IONs. Biomineralization refers to the crystallization or precipitation of an inorganic material within or around a cell or organism by precise control of various factors, including ion concentration, nucleation process, pH, and redox potential in magnetic protein nanocages, which are usually accomplished via compartmentalization. Therefore, a variety of compartmentalized protein cages have been extensively explored for the formation of nanoparticles with the length scale of 5–30 nm, such as the DNA protection during starvation (Dps) proteins, ferritin, encapsulin, and so on. For instance, in our previous work, we synthesized ultrasmall copper sulfide nanoparticles using ferritin nanocages by a biomimetic synthesis method.Citation4 Taking advantages of the strong near-infrared absorbance and high photothermal conversion efficiency, they have been successfully applied for photothermal therapy. Moreover, a nanocomposite was developed based on arginine‐glycine‐aspartate (RGD)-modified ferritin nanocages and sinoporphyrin sodium, which has been successfully used for photothermal and photodynamic therapy.Citation5 Furthermore, as the largest bacterial iron-storage organelle, encapsulin was introduced as a self-assembling nanosphere system and found exhibiting the ferroxidase activity, allowing efficient intraluminal iron sequestration, which could possibly enable magnetic resonance imaging (MRI).Citation6 With the instinct feature of these protein nanocages, they are promising to be engineered for a wide range of nanobiotechnology applications.Citation7,Citation8 However, these magnetic protein nanocages have barely been investigated as MHT agents.
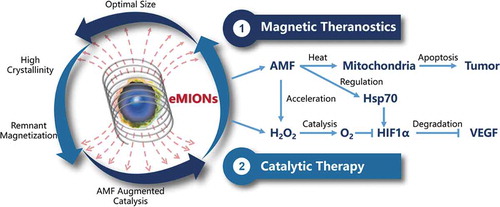
In our most recent study, encapsulin protein nanocages were genetically engineered to synthesize high-quality iron oxide nanocomposites via a biomineralization procedure.Citation9 Such an efficient and facile strategy obtained the encapsulin-produced magnetic iron oxide nanocomposites (eMIONs) presenting advanced features in size, composition, magneto-crystalline anisotropy, and magnetization effects for cancer therapeutics (). On one hand, eMIONs possessed desirable magnetic saturation and remnant magnetization properties, leading to superior magnetic-to-thermal conversion efficiency and specific absorption rate for magnetic theranostics. On the other hand, eMIONs showed enhanced catalase-like activity in the mild acidic environment, the efficiency of which could be improved significantly under AMF, achieving synergy effects for MHT. Taking together, considering the excellent performance of eMIONs in MRI-guided magneto-catalytic combination therapy both in vitro and in vivo, it has been demonstrated that eMIONs not only could provide valuable insights for the biomimetic development of a promising MHT agent, but also have great potential for profound applications in clinical cancer theranostics, which can be expected to be a new breakthrough in cancer treatment. Although significantly enhanced theranostic performance has been obtained by applying eMIONs in subcutaneous tumor and orthotopic hepatocellular carcinoma, further research and clinical trials in various kinds of diseases/conditions may well be required to expand the potential biofunctions of eMIONs for magnetic hyperthermia cancer therapy.
Figure 1. Magneto-catalytic theranostics of encapsulin-produced magnetic iron oxide nanocomposites (eMIONs). Schematic illustration of eMIONs for magnetic-to-thermal conversion under alternating magnetic field (AMF). With the optimal size of the inner cores for magnetic hyperthermia induction, ~100% crystallinity, excellent magnetic saturation and remnant magnetization, eMIONs exhibit superior magnetic thermal induction under AMF for magnetic theranostics. Moreover, eMIONs are powerful catalase-like nanozymes for catalytic therapy. It can suppress the tumor growth effectively by generating heat and inducing apoptosis, as well as synergize the catalyzation by augmenting oxygen production to modulate the tumor microenvironment, activating heat-shock proteins (Hsp) 70 and decreasing the expression of hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VEGF)
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors are grateful for the support of the Major State Basic Research Development Program of China (2017YFA0205201), the National Natural Science Foundation of China (82003517, 81925019, and U1705281), the Fundamental Research Funds for the Central Universities (20720190088 and 20720200019), and the Program for New Century Excellent Talents in University, China (NCET-13-0502).
References
- Mahmoudi K, Bouras A, Bozec D, Ivkov R, Hadjipanayis C. Magnetic hyperthermia therapy for the treatment of glioblastoma: a review of the therapy’s history, efficacy and application in humans. Int J Hyperthermia. 2018;34(8):1–2. doi:10.1080/02656736.2018.1430867.
- Liu X, Zhang Y, Wang Y, Zhu W, Li G, Ma X, Zhang Y, Chen S, Tiwari S, Shi K. Comprehensive understanding of magnetic hyperthermia for improving antitumor therapeutic efficacy. Theranostics. 2020;10(8):3793. doi:10.7150/thno.40805.
- Zeth K, Hoiczyk E, Okuda M. Ferroxidase-mediated iron oxide biomineralization: novel pathways to multifunctional nanoparticles. Trends Biochem Sci. 2016;41(2):190–203. doi:10.1016/j.tibs.2015.11.011.
- Wang Z, Huang P, Jacobson O, Wang Z, Liu Y, Lin L, Lin J, Lu N, Zhang H, Tian R. Biomineralization-inspired synthesis of copper sulfide–ferritin nanocages as cancer theranostics. ACS Nano. 2016;10(3):3453–3460. doi:10.1021/acsnano.5b07521.
- Huang C, Chu C, Wang X, Lin H, Wang J, Zeng Y, Zhu W, Wang Y-XJ, Liu G. Ultra-high loading of sinoporphyrin sodium in ferritin for single-wave motivated photothermal and photodynamic co-therapy. Biomat Sci. 2017;5(8):1512–1516. doi:10.1039/C7BM00302A.
- Sigmund F, Pettinger S, Kube M, Schneider F, Schifferer M, Schneider S, Efremova MV, Pujol-Martí J, Aichler M, Walch A. Iron-sequestering nanocompartments as multiplexed Electron Microscopy gene reporters. ACS Nano. 2019;13(7):8114–8123. doi:10.1021/acsnano.9b03140.
- Lei Z, Wang J, Lv P, Liu G. Biomimetic synthesis of nanovesicles for targeted drug delivery. Sci Bull. 2018;63(11):663–665. doi:10.1016/j.scib.2018.05.001.
- Wang Z, Xu L, Yu H, Lv P, Lei Z, Zeng Y, Liu G, Cheng T. Ferritin nanocage-based antigen delivery nanoplatforms: epitope engineering for peptide vaccine design. Biomat Sci. 2019;7(5):1794–1800. doi:10.1039/C9BM00098D.
- Zhang Y, Wang X, Chu C, Zhou Z, Chen B, Pang X, Lin G, Lin H, Guo Y, Ren E. Genetically engineered magnetic nanocages for cancer magneto-catalytic theranostics. Nat Commun. 2020;11(1):1–11. doi:10.1038/s41467-020-19061-9.
