ABSTRACT
Aneuploidy, the presence of an aberrant number of chromosomes, has been associated with tumorigenesis for over a century. More recently, advances in karyotyping techniques have revealed its high prevalence in cancer: About 90% of solid tumors and 50–70% of hematopoietic cancers exhibit chromosome gains or losses. When analyzed at the level of specific chromosomes, there are strong patterns that are observed in cancer karyotypes both pan-cancer and for specific cancer types. These specific aneuploidy patterns correlate strongly with outcomes for tumor initiation, progression, metastasis formation, immune evasion and resistance to therapeutic treatment. Despite their prominence, understanding the basis underlying aneuploidy patterns in cancer has been challenging. Advances in genetic engineering and bioinformatic analyses now offer insights into the genetic determinants of aneuploidy pattern selection. Overall, there is substantial evidence that expression changes of particular genes can act as the positive selective forces for adaptation through aneuploidy. Recent findings suggest that multiple genes contribute to the selection of specific aneuploid chromosomes in cancer; however, further research is necessary to identify the most impactful driver genes. Determining the genetic basis and accompanying vulnerabilities of specific aneuploidy patterns is an essential step in selectively targeting these hallmarks of tumors.
1. Introduction
In 1914, Theodor Boveri formulated the first hypothesis linking incorrect chromosome content with the origin of tumorigenesis based on observations made by pathologist David Paul von Hansemann at the end of the 19th century.Citation1 However, just how widespread aneuploidy is across many different tumor types has only been appreciated since the development of high throughput karyotyping techniques.Citation2–6 Approximately 90% of solid tumors and 50–70% of hematopoietic cancers display chromosome gains and/or losses.Citation7 When analyzed at the level of specific chromosomes, there are strong patterns that are observed in cancer karyotypes both pan-cancer and for specific cancer types. We use the term aneuploidy patterns to refer to copy number alterations of whole chromosomes or chromosome arms that occur at a high frequency relative to other chromosomes/arms. Strong aneuploidy patterns point toward the selection of specific aneuploid chromosomes in cancer. Such aneuploidy patterns are observed in all stages of tumorigenesis, suggesting important roles in cancer development and progression.
Although specific chromosomal arm aneuploidies rank amongst the most frequent alterations in cancer, determining the consequences of aneuploidy on cancer development has been hindered by the difficulty in engineering specific aneuploidies. Furthermore, the impact of aneuploidy on subtle expression changes of hundreds of genes simultaneously has made it difficult to determine the genetic basis behind their selection. However, recent advances in both genetic engineering of aneuploidy in human cells and bioinformatic analyses of cancer genomes have led to substantial insights into the genetic determinants of the selection of aneuploidy patterns in human cells.Citation6,Citation8–13 Determining the mechanistic basis of aneuploidy patterns will lead to a better understanding of cancer and has the potential to identify new targets for personalized cancer treatments.
In this review, we will describe the types of aneuploidy patterns observed in cancer and discuss the selection forces that potentially determine these patterns. The cellular mechanisms leading to aneuploidy formation, the general consequences of aneuploidy, and methods for engineering aneuploidy have been extensively reviewed elsewhere (compareCitation14–17 and are not the focus of this review.
2. Types of aneuploidy patterns in cancer
Specific chromosome gains and losses are among the most common genomic alterations in cancer. For comparison, mutation of the tumor suppressor gene TP53 is the most prevalent alteration identified within The Cancer Genome Atlas (TCGA) dataset, at 37%.Citation18 The next 23 most common alterations observed in TCGA tumors are chromosome arm aneuploidies.Citation13 The most common of these chromosome arm alterations include the gains of 20q (31.9%), 7p (31%), 8q (29.6%), 1q (27.8%), 7q (27.3%), and 20p (26.4%), and the loss of 17p (24.6%).Citation13 These frequencies are comparable to those measured in other cancer data sets.Citation3,Citation4,Citation19,Citation20
Another important aspect of aneuploidy patterns lies in the specificity of chromosome gains vs. losses. Across cancer-types, most chromosome arms are either gained or lost, but rarely both.Citation3 For instance, chromosome arm 20q, the most prevalent gained chromosome arm encompassing 31.9% of samples, is decreased in copy number in only 1.2% of tumors. Likewise, the most common chromosome arm loss, 17p, prevalent in 24.6% of TCGA tumors, is gained in a mere 5.2% of tumors,Citation13 (). This trend toward either chromosome gain or loss suggests a strong selection bias, as any given chromosome missegregation event would create one daughter cell with an extra copy and one daughter cell lacking one copy of the chromosome. These pan-cancer aneuploidy patterns indicate that copy number changes of specific chromosomes play an important role in carcinogenesis across many different cancer types and microenvironments.Citation21 However, it is not known whether particular aneuploid chromosomes are selected for in different cancer types for the same underlying reasons.
Figure 1. Pan-cancer and cancer type-specific aneuploidy patterns a. Frequency of chromosome arm aneuploidies from most to least common across all TCGA cancer types. The frequencies of cancer type-specific aneuploidy patterns highlighted in b are depicted in light blue and light red. The directionality of aneuploidy patterns (gain vs. loss) for chromosome arms 20q and 17p are highlighted. 20q is the most commonly gained and most infrequently lost chromosome arm, whereas 17p is the most frequently lost chromosome arm and only rarely gained. Bars for chromosome arm gains are red and losses are blue. Frequencies of chromosome arm aneuploidies are taken from reference.Citation13 b. Heat map of the average copy number data for somatic chromosome arms of BRCA (Breast Invasive Carcinoma), COAD (Colon Adenocarcinoma), and GBM (Glioblastoma Multiforme) cancers from the TCGA data set. Copy number gains are red and copy number losses are blue. Frequencies are taken from reference.Citation8 Characteristic cancer type-specific aneuploid chromosomes are surrounded by a black square.
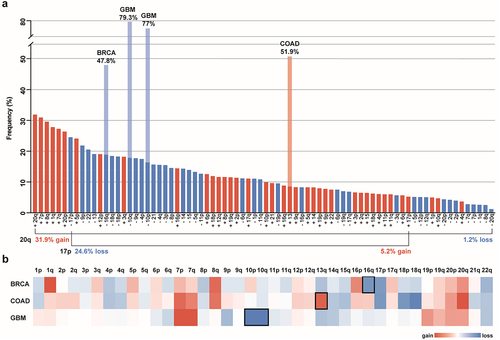
Aneuploidy patterns for individual cancer types can differ significantly from pan-cancer patterns in both the percentage and the directional bias,Citation3,Citation21,Citation22 (). One example of cancer type-specific aneuploidy patterns is for chromosome 13. Although chromosome 13 is often decreased in copy number across most cancer types, colorectal and stomach cancers frequently gain chromosome 13 instead.Citation6,Citation23 For other cancer types, certain aneuploidies are considered a hallmark of the disease. The clearest example of this is in glioblastoma, where chromosome 7 gain and 10 loss are each found in ~ 70–90% of cases.Citation24 Aneuploidies are also indicators of cancer subtypes. For example, chromosome 16q loss is observed in the majority of differentiated breast cancer, but only rarely in the undifferentiated form.Citation25 This cancer type specificity is similar to what is observed for mutations in many oncogenes (OGs) and tumor suppressor genes (TSGs), as certain aneuploidies are observed across many different cancer types while others are more cancer-type specific.Citation26 The identification of different aneuploidy patterns points toward a context-dependent selective advantage of aneuploidy-induced changes in tumor cells.
3. Specific aneuploidy patterns have been identified at all stages of carcinogenesis
Recently, progress has been made in determining the timing of mutational events in cell transformation. Top-down studies aiming to determine the evolutionary history of a cancer based on karyotype heterogeneity have concluded that aneuploidy patterns are present in all stages of oncogenesis and continue to change over time.Citation27,Citation28 These evolutionary reconstructions indicate that there are multiple roles of aneuploidies in carcinogenesis (). In this section, we discuss aneuploidy patterns observed in tumor initiation, progression, metastasis, and therapy resistance. We will also examine the evidence for these aneuploidy patterns having a causative role in carcinogenesis. The subsequent section will elaborate on potential genetic mechanisms responsible for these frequent aneuploidies.
Figure 2. Aneuploidy patterns have been identified in all stages of cancer. Schematic of identified aneuploidy patterns in cancer or cell lines in the context of cancer evolution. The gray boxes display examples of recurring aneuploidy patterns. Boxes with dashed lines represent correlative evidence; boxes with solid lines represent direct evidence based on cell culture experiments. GBM: Glioblastoma Multiforme, ccRCC: clear cell Renal Cell Carcinoma, FHWT: Favorable Histology Wilms Tumor, BRCA: Breast Invasive Carcinoma, HCC: Hepatocellular Carcinoma, COAD: Colon Adenocarcinoma, HNC: Head and Neck Cancer, NSCLC: Non-Small Cell Lung Cancer.
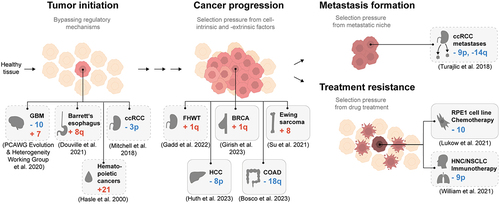
3.1. Aneuploidy patterns in tumor initiation
The presence of aneuploidy in precancerous lesions of certain cancer types raises the possibility for a causative role in tumor initiation. In a study examining tumor samples of patients with Barrett’s esophagus, investigators identified the gain of chromosome 8q in 75% of the cases (N = 20).Citation29 Chromosome 8q gain may result from the CIN inducing stressful microenvironment attributable to acid reflux and, together with other cellular changes, contribute to cancer initiation. Abe and colleagues identified that the exposure of a Barrett’s esophagus metaplastic cell line, CP-A, to acids and deoxycholic acid resulted in the hypomethylation of α-satellite DNA. This lead to heightened expression of non-coding RNA transcribed from α-satellite sequences ultimately resulting in chromosomal instability.Citation30 However, it should be noted that in their study following acid treatment 8q gain was not among the acquired aneuploidies, suggesting that the acidic conditions may be responsible for increasing the amount of aneuploidy but not the selection of 8q.
Various groups have modeled the progression of cancers over time to determine which changes to the genome act early in their evolution. Modeling of the somatic evolution of clear cell renal cell carcinoma (ccRCC) revealed that chromosome 3p is frequently lost early in oncogenesis (30–40% of 95 biopsies). Estimates date this event back as far as 30–50 years before cancer diagnosis.Citation31 In another study, investigators calculated that chromosome 7 gain and 10, 17p, and 9p loss typically occur very early in the development of glioblastoma, based on the low number of single nucleotide variants (SNV) on chromosomes before amplification and an assumed mutation rate of SNV per base pair and cell division.Citation28 In agreement with this, another research group comparing matched primary and recurred glioblastomas calculated that 7 gain and 9p and 10 loss are acquired 2–7 years before cancer diagnosis.Citation32
Further evidence for aneuploidy playing a causative role in cancer formation comes from studies on trisomy 21. Individuals with an extra copy of chromosome 21 have a substantially increased risk of developing hematopoietic cancers, but, conversely, the risk for developing solid tumors is significantly decreased.Citation33,Citation34 Interestingly, trisomy 21 is the most frequent aneuploidy in blood cancers from euploid individuals.Citation35,Citation36 These observations together suggest that the gain of chromosome 21 contributes to the development of hematopoietic tumorigenesis.
Experiments in mice have shown that despite mostly having a negative effect on cellular fitness, aneuploidy can promote tumorigenesis. For instance, Williams and colleagues determined that the effect of aneuploidy on transformation capacity in mouse embryonic fibroblasts is specific to the identity of the aneuploid chromosome, i.e. trisomy 13 inhibiting, trisomy 19 neutral and trisomy 16 supporting transformation.Citation37 Mouse models for increased chromosome missegregation through centrosome amplification, kinetochore defects, or decreased spindle assembly checkpoint activity have demonstrated an increased incidence of tumor formation, suggesting a role for either chromosome instability (CIN) or aneuploidy in this process.Citation38–43 Furthermore, for certain cancer types, this resulted in strong aneuploidy patterns, suggesting that particular chromosomes are acting as cancer drivers.Citation38,Citation44 However, the causative role of individual aneuploidies as a primary source of tumor initiation is still under debate, as engineered aneuploidy typically leads to decreased proliferation, transformation, and tumor growth.Citation37,Citation45,Citation46
3.2. Aneuploidy patterns in cancer progression
In patient survival analyses comparing cancer karyotypes, higher levels of aneuploidy generally correlate with poor prognosis.Citation47 Particular aneuploid chromosomes are mostly associated with unfavorable outcomes (87%); however, the remaining aneuploidies (13%) are associated with increased patient survival. For example, although likely rare, glioblastomas with 10p gain have a more beneficial prognosis than glioblastomas without this specific aneuploidy.Citation48 Intriguingly, certain combinations of frequent aneuploidies have also been found to correlate with patient survival, suggesting a more complex relationship between aneuploidy and cancer progression.Citation48,Citation49 For instance, the coincident loss of chromosome arms 9p and 21q in liver hepatocellular carcinoma is predictive for a decreased disease free survival.Citation49 Notably, cancer karyotypes often have multiple aneuploidies, and therefore combinations of aneuploidies may have an increased potential to drive tumor progression.
Even though certain aneuploidies accumulate in cancer cells at high rates, that does not necessarily mean that they directly contribute to cancer progression. Attempts to determine if specific aneuploid chromosomes contribute to tumor formation have yielded largely negative results. Mouse xenografts of human cancer cells engineered with frequently observed aneuploidies generally do not promote tumor formation.Citation45–48 Furthermore, when the tumors that do form are resequenced, the engineered aneuploidy is frequently lost, indicating that it was acting in an inhibitory manner. These results highlight the context-sensitive nature of aneuploid chromosome selection in cancer.
Although recapitulating the advantages of pan-cancer aneuploidy patterns has been challenging, there are a few cases in which a cancer type-specific advantage of specific aneuploidies could be demonstrated in cell culture. For instance, the gain of chromosome 1q is very frequently acquired in breast cancer, among other types. Using breast cancer cell lines with and without 1q gain, Girish and colleagues could show that xenograft growth and anchorage-independent growth is dependent on the 1q gain.Citation11 Similarly, the gain of chromosome 8 is prevalent in multiple types of cancer. In a Ewing sarcoma cell line harboring the specific gene fusion EWS-FLI1, Su and colleagues determined the cancer type-specific benefit of this aneuploidy in restoring DNA replication fidelity.Citation50 In another study, liver cancer cell lines engineered with monosomy for chromosome arm 8p had accelerated migratory and invasive potential.Citation12 Finally, Bosco and colleagues showed that the engineered loss of chromosome 18q, which occurs frequently in colorectal cancer, reduced the sensitivity to growth-inhibition by TGF-β.Citation9 These bottom-up approaches highlight the complexity of the role of aneuploidies in cancer progression.
3.3. Aneuploidy patterns involved in resistance to immunotherapy and chemotherapy
The aspect of tumor progression that is most highly associated with aneuploidy is immune evasion and resistance to immunotherapy.Citation51 In general, highly aneuploid tumors are associated with poor prognosis after immunotherapy.Citation52,Citation53 More recently, the loss of chromosome 9p, which is common in multiple cancer types, has been linked to immune evasion and immunotherapy resistance. In HPV negative head and neck cancer (HNC) and Non-Small Cell Lung Cancer (NSCLC), the loss of chromosome arm 9p underlies a switch from an immune hot to an immune cold tumor microenvironment and serves as a predictive marker associated with limited clinical benefit from anti-PD-(L)1 therapy.Citation54 Overall aneuploidy levels and specific aneuploidies both correlate with immune evasion and immunotherapy resistance, making aneuploidy a promising clinical prognostic biomarker for immunotherapy.
However, it is still unclear if the immune evasion associated with aneuploidy primarily results from the aneuploidy itself, or from chromosomal instability. Certain types of CIN can generate cytosolic DNA, which triggers interferon-STAT1/3 and TNF – NF-κB immune signaling through the cGAS/STING pathway. In advanced stages of tumorigenesis, an adaptive reconfiguration of signaling pathways downstream of STING enables tumors to avoid the pro-inflammatory effects of type I interferons, resulting in immune evasion.Citation55–57
The frequency of aneuploidies have also been observed to increase following chemotherapies. For instance, in Favorable Histology Wilms Tumors (FHWT), the gain of chromosome 1q (28% of individuals) has been linked with reduced overall survival. Gadd and colleagues identified that the number of samples with gain of chromosome 1q was significantly higher (74.5%, N = 51) in relapse samples compared to primary samples (47%, N = 45), indicative of a role of 1q in chemotherapy resistance in FHWT.Citation58
Recent experiments identified the acquisition of individual aneuploidies as resistance mechanisms to chemotherapeutic drugs in cell culture.Citation59,Citation60 By adapting RPE1 cells to the chemotherapeutic drug paclitaxel after a pulse of a spindle assembly checkpoint inhibitor to induce aneuploidy, Lukow and colleagues identified the loss of one copy of chromosome 10 in separately adapted populations.Citation60 In a study from our lab, we demonstrated that prolonged inhibition of the spindle assembly checkpoint in multiple non-transformed and cancer cell lines resulted in the selection of distinct aneuploidy patterns that correlated with increased drug resistance.Citation8 In both studies, cell lines harboring the individual frequent monosomies were sufficient for drug resistance. Collectively, there is substantial evidence from evolution experiments in cell culture and cancer karyotype analysis demonstrating that aneuploidy patterns contribute to the acquisition of resistance phenotypes in cancer cells.
3.4. Aneuploidy patterns in metastases
In general, metastatic tumors have a substantially higher degree of aneuploidy than primary tumors,Citation61,Citation62 with a median copy number change of ~ 5 chromosome arms compared to ~ 3.Citation49 The increase in aneuploidy following metastasis also occurs for specific chromosomal aneuploidies. For primary ccRCC, 9p and 14q loss were observed in most of the analyzed metastatic sites, which indicates a selective advantage of these aneuploidies for metastasis development.Citation63 In a mouse model of metastatic renal cell carcinoma with partial 9p loss, Perelli and colleagues demonstrated that this evolutionary pressure leads to recurrent copy number alterations. In particular, the loss of mouse chromosome 16q along with the initial 9p loss results in a proliferative advantage.Citation64
Metastasis is one of the few areas where the engineering of specific chromosome gains has an effect on promoting tumor formation in mouse xenografts. Human colon carcinoma cells (HCT116) engineered with trisomy of chromosome 5 displayed significantly increased invasiveness in comparison to their near-diploid progenitor cells, suggesting a role for this aneuploidy in metastasis.Citation48 In conclusion, aneuploidy levels increase substantially after metastasis, and there are a few examples that indicate a selective advantage of specific aneuploidy patterns for cell invasion and metastasis.
Overall, there is substantial evidence that aneuploidy patterns play multiple roles in tumor evolution. There are strong correlations between specific aneuploidies and outcomes for tumor initiation, progression, metastasis formation, immune evasion and resistance to therapeutic treatment. In addition to aneuploidy’s overall impact on cancer cells, the high prevalence of specific chromosomal changes indicates positive selection and underscores the important role that aneuploidy patterns play in tumor evolution.Citation65 However, the genetic mechanisms underlying the selection of these aneuploidy patterns are often unclear.
4. What is the genetic basis behind aneuploidy patterns?
Many different theories have been proposed for the mechanisms underlying the frequent aneuploidy observed in cancer. Given the aneuploidy patterns described above, we will focus on theories related to chromosome-specific effects. The prevalence of specific aneuploidies in cancer is not primarily determined by different missegregation rates between chromosomes. Measurements of individual missegregation rates found, at most, a ~ 4-fold difference between chromosomes,Citation66,Citation67 which cannot account for the differences in frequencies observed in cancer. Additionally, chromosomes with the highest missegregation rates do not correlate with those with the highest aneuploidy rates in cancer. Larger chromosomes missegregate more frequently, whereas, if anything, the opposite trend is seen in aneuploidy frequency.Citation35,Citation66,Citation67 Furthermore, symmetric increases in both chromosome gain and loss would be expected for differences in missegregation rates.
The bias of chromosomes toward being either gained or lost implies that the respective up- or downregulation of specific genes on the aneuploid chromosome drives the observed directional aneuploidy patterns. In agreement with this, measurements of mRNA and protein levels in aneuploid cells show that expression often scales with copy number.Citation68–71 However, some degree of dosage compensation does occur for genes on aneuploid chromosomes. In yeast, human cell lines, and tumor cells, ~20–70% of genes display at least partial dosage compensation at the protein level and 5–10% are present at near euploid levels.Citation68,Citation72,Citation73,Citation74 Whether an aneuploidy will be selected for is likely a balance between all of the positive and negative effects of the up- or downregulation of hundreds of genes across the chromosome in a particular cellular and environmental context.
4.1. Positive selection of aneuploidy patterns
Cancer cells are characterized by their sustained proliferation. Genes that promote proliferation and are often found to be overactivated in cancer are referred to as OGs, while genes that suppress proliferation and are often deactivated in cancer are termed TSGs. Computational analyses of the TCGA have revealed a correlation between the presence of OGs and TSGs on a chromosome and the frequency and directionality (gain vs. loss) of copy number alterations in cancer. OGs are overrepresented on predominantly gained chromosomes and TSGs on lost chromosomes.Citation75 In addition, a gain-of-function screen for regulators of proliferation identified cancer type-specific proliferation driver genes present on chromosomes that are frequently gained in a tissue type-specific manner, suggesting that proliferation drivers are cancer type-specific.Citation76 Together these studies indicate that the copy number changes of proliferation-associated genes are correlated with, and likely contribute to common and cancer type-specific aneuploidy patterns. contains a summary of frequently aneuploid chromosomes and potential proliferation-related driver genes. However, for most of the genes in , there is no experimental evidence showing that one copy number change is sufficient for conferring a cancer-related phenotype. In addition, dosage compensation of OGs and TSGs on aneuploid chromosomes may complicate the identification of the driver genes. For instance, in cancer cell lines with gain of chromosome 8q, MYC expression was frequently buffered and only showed substantial overexpression upon focal gain.Citation73 To date, there are no candidate genes yet identified for many aneuploidy patterns in specific cancer types.
Table 1. List of putative driver genes of aneuploidy patterns for the 10 most common pan-cancer chromosome arm aneuploidies. Hallmark OGs and TSGs from the Catalogue of Somatic Mutations in Cancer (COSMIC) database with an aneuploidy frequency above 25% in the indicated tumor type.
Recent evidence highlights the context-dependent nature of aneuploidy formation. An analysis of the lineage-specific recurrence of partial chromosome gains and losses shows that cancer type-specific driver genes underly the selection of pan-cancer aneuploidy patterns. For instance, monosomy of chromosome arm 3p displays different regions driving positive selection across tumor types.Citation13 These data support the possibility that multiple different cancer type-specific drivers underly selection, even for pan-cancer aneuploidy patterns.
Although direct evidence for specific genes contributing to the selection of aneuploidies in cancer is rare, there are many examples in model systems. The first observation of this phenomenon was in the pathogenic yeast Candida albicans, where drug resistance was conferred by the gain of extra copies of the left arm of chromosome 5, containing the resistance genes ERG11 and TAC1.Citation77,Citation78 Additionally, many examples of obtaining resistance to stress conditions through specific aneuploidy chromosomes have been identified in the nonpathogenic yeast Saccharomyces cerevisiae (reviewed in Citation79). For example, adaptation of haploid yeast cells to mutations that induce high rates of chromosome missegregation led specifically to the frequent gain of 5 different chromosomes in the adapted cells. Relocating one gene, SLI15INCENP, from its endogenous chromosome to a different chromosome was sufficient to alter the aneuploidy pattern of adapted cells toward gaining the alternate chromosome.Citation80 In mice, clonal selection of lymphomas led to the recurrent acquisition of chromosome 15 gain, which contains the oncogene Myc. Expression of human MYC on chromosome 6 resulted in the additional selection of this chromosome in lymphomas.Citation44 For the chromosome 9p monosomy that underlies defects in anti-tumor immunity, a mouse model of pancreatic cancer was used to identify the loss of type 1 interferon genes contributing to the phenotype.Citation81 These and other studies in model organisms demonstrate that the expression changes of specific genes contribute to the selection of aneuploid chromosomes.
Recently, progress has been made in identifying specific genes that at least partially contribute to aneuploidy-dependent advantages in certain cancer cell lines. In one study, the authors restored trisomic chromosome arms to diploid copy number levels in human cancer cell lines, and could subsequently observe reduced growth both in culture and in mouse xenographs.Citation11 The p53 negative regulator MDM4 was identified as a contributor to the growth advantage of chromosome 1q gain. Interestingly, the authors also found a negative correlation between 1q gain and p53 mutation, suggesting that 1q gain could potentially act as an alternative pathway for p53 inactivation. However, the elimination of other common aneuploidies displayed little-to-no changes in phenotype, highlighting the difficulties in determining the basis for aneuploid chromosome selection.Citation11 Another study investigating the selection of 1q gain in breast cancer identified increased Notch-signaling as a driver underlying the gain of the chromosome arm. In both mammary epithelial cell lines and mammary tumors with 1q gain, they found increased expression of the γ-secretase genes APH1A, NCSTN, and PSEN2. Expression levels of NCSTN, present on 1q, positively correlated with Notch activation.Citation82 Together, these two studies suggest that 1q could be gained for multiple independent reasons. The proliferative advantage of chromosome 8 trisomy in the context of the oncogenic EWS-FLI1 fusion gene could be partially attributed to the copy number gain of the gene for the cohesin subunit RAD21. Mild RAD21 overexpression partially relieves the replication stress caused by the oncogenic fusion protein.Citation50 Recent studies have also shed light on the genetic link between trisomy 21 and hematopoietic cancers, pointing toward the dysregulation of RUNX1 and likely other genes on chromosome 21 contribute to the early stages of hematopoiesis.Citation83,Citation84
Overall, there is substantial evidence that expression changes of particular genes can act as the positive selective forces for evolving through aneuploidy. However, in the majority of cancer-associated aneuploidies, it is not clear which genes are the source of the selection, nor if the same genes are providing the selective force in different cancer types.
4.2. Negative selection of aneuploidy patterns
Negative selection of gene mutations is generally believed to be a negligible force on tumor growth, as the majority of mutations in the cell will be silent or suppressed by the homologous allele.Citation85,Citation86 By contrast, aneuploidies affect hundreds of genes all at the same time, which can substantially impact the fitness of the cells. The genetic imbalance of somatic aneuploidy leads to a reduced fitness in every species studied.Citation37,Citation69,Citation87 Furthermore, there are strong negative correlations between fitness and the number of genes on aneuploid chromosomes,Citation87,Citation88,Citation89 indicating that the imbalanced expression of genes underlies the growth defects. Besides defective growth, aneuploidy is associated with genomic instability, replication stress, proteotoxic stress, DNA damage and apoptosis.Citation90,Citation91,Citation92,Citation93,Citation94 This is consistent with the observation that aneuploidy is very rare in healthy tissue.Citation95 Additionally, only three autosomal germline aneuploidies are compatable with live births, the gains of chromosome 13, 18 and 21. These three chromosomes have the fewest number of genes. All other autosomal aneuploidies lead to early spontaneous abortion.Citation96
In some studies, the correlation between chromosome gene number and negative selection is also seen in cancer. For ovarian carcinoma, the frequency of losing or gaining a chromosome arm negatively correlates with the number of genes on the chromosome arm.Citation97 In solid tumors, the number of genes on chromosomes inversely correlates with the percentage of chromosomes being lost, indicating that the loss of gene-rich chromosomes is under negative selection pressure.Citation35 These observations support the hypothesis that aneuploidies are negatively or positively selected based on the balance between beneficial and detrimental consequences of the induced gene copy number alterations.
These negative selection forces from gene expression changes could either be general effects on cellular fitness or specifically inhibit cancer progression. Genes that are negatively selected due to decreased fitness would be either haploinsufficient for chromosome loss or triplosensitive for chromosome gain. Intriguingly, a study in yeast found that haploinsufficient genes are frequently triplosensitive as well, suggesting that certain genes have an narrow range of optimal expression.Citation98 Although haploinsufficiency and triplosensitivity of individual genes rarely lead to strong phenotypes at the cellular level, the additive effects of many such genes across an entire chromosome likely lead to the observed growth defects.Citation99 In humans, an extensive meta-analysis of rare copy number variations from over 950.000 individuals spanning 54 disease phenotypes identified 3,635 dosage sensitive protein-coding genes.Citation100
As mentioned above, there is a correlation between OGs on a chromosome and how frequently it is gained and TSGs and how frequently it is lost.Citation75 Intriguingly, the reverse correlations also exist. OGs negatively correlate with chromosome loss and TSGs negatively correlate with chromosome gain. This points toward cancer-specific sources of negative selection. This could result from cancer progression being inhibited by the overexpression of TSGs on chromosome gains and the decreased expression of OGs on chromosome losses. For some tumor suppressor genes, overexpression reduces proliferation, transformation, and or malignancy. Overexpression of the TSG PTEN has been shown to reduce the amount of tumor growth in a mouse mammary cancer model of Wnt-1 overexpression.Citation101 Similarly, overexpression of the TSG RB1 inhibits tumor progression in metastatic melanoma cells.Citation102 Since many proto-oncogenes are involved in promoting cell cycle entry, they would therefore potentially decrease proliferation when decreased in expression.
As mentioned above, engineered aneuploid chromosomes in human cancer cells revert to euploidy following mouse xenografts, suggesting that these negative selective pressures occur in cancer as well. Recent work from Shih and colleagues in which they analyzed the length distribution of large partial arm somatic copy number alterations (SCNAs) from the TCGA provides evidence for the existence of specific regions of aneuploid chromosomes under negative selection in cancer. Out of 193 loci identified as being under selection, 103 were categorized to be under negative selection.Citation13 Unlike with other types of genomic alterations, there are substantial data demonstrating negative selection of aneuploid chromosomes. It is therefore surprising that aneuploidy is so common in cancer, and the forces of positive selection must be especially strong to overcome the negative selection.
4.3. Selection forces underlying aneuploidy patterns that can be either positive or negative
Many types of changes to the genome can either promote or inhibit growth depending on the context. For example, multiple aneuploid chromosomes within the same cell have the potential to affect each other in complex ways. Evidence for this comes from positive and negative correlations between specific aneuploid chromosomes in cancers.Citation49,Citation80,Citation103 Such correlations have also been observed in cellular adaptation assays. For instance, chronic myeloid leukemia cells adapted to the drug reversine through the acquisition of many frequent aneuploidies; however, the gain of chromosome 7 strongly negatively correlated with the loss of chromosome 14. Over time, chromosome 14 loss became much more frequent as chromosome 7 gain was lost from the cell populations. These results suggest negative genetic interactions between aneuploid chromosomes.Citation8 Direct evidence for such interactions between specific aneuploid chromosomes comes from experiments using budding yeast. By examining the karyotypes of yeast after adaptation to the high rates of chromosome missegregation, we previously identified specific positive and negative correlation between aneuploid chromosomes. Engineered yeast strains with chromosome gain combinations showed growth rates that differed from what was predicted by the growth of each individual aneuploid chromosome on its own.Citation80 In summary, genetic interactions between aneuploid chromosomes provides a plausible mechanism for positive as well as negative selection of aneuploidy patterns in cancer.
Loss of heterozygosity (LOH) occurs when a heterozygous genotype becomes homozygous through the loss or replacement of one of the two copies. In the case of aneuploidy, loss of one of the two copies of a chromosome eliminates all heterozygosity across its length. In addition to copy-number loss LOH, the chromosome can be subsequently duplicated, resulting in copy-number-neutral LOH or even amplified resulting in gain-of-copy LOH.Citation104,Citation105 A recent study suggests that across the 33 TCGA tumor types an average of 16% of genes display LOH, mostly from copy-number-loss LOH. This high frequency of LOH suggests a relevant mechanism in cancer evolution for positively selecting chromosome loss.Citation105 LOH of chromosome arms 18p, 17p, 10p, 4p, 18q, 9p and 8p is frequent in cancer and TSGs are enriched in LOH regions.Citation104 Conversely, LOH can be under negative selection. The most extreme example of this would be for recessive loss-of-function alleles of essential genes that are uncovered by chromosome loss. The negative effects of LOH on specific genes could also be potential targets for cancer therapy.Citation105 One study determined the presence of negative selection copy number loss of genomic regions where harmful mutations cannot be balanced out by healthy allele copies.Citation106
4.4. Neutral selection of aneuploidy patterns
Neutral selection is thought to be acting on most of the acquired gene mutations in cancer, which are referred to as passenger mutations.Citation86 However, neutral selection for aneuploidy would seem unlikely due to the expression changes of hundreds of genes at once as described above. However, one candidate for neutral selection shaping aneuploidy patterns could be the loss of the sex chromosomes in specific cancer types. In the TCGA, 1504 of 5014 analyzed male tumors (30%) exhibited complete or relative loss of Y (LOY), ranging from 80% in kidney renal papillary cell carcinoma (KIRP) to 1.3% in Pheochromocytoma and Paraganglioma.Citation107 In most analyzed cancer types LOY correlated with aneuploidy rates as a whole, suggesting neutral selection. However, at least three tumor types – KIRP, uveal melanoma (UVM, 47% LOY) and kidney renal cell carcinoma (KIRC, 42% LOY) – have especially high frequencies of LOY, suggesting positive selection.Citation107 Additionally, loss of the Y chromosome in bladder cancer has recently been demonstrated to drive evasion of the adaptive immunity.Citation108 Therefore, recurrent aneuploidy of even the smallest chromosome likely has a role in tumor development. The loss of the X chromosome (LOX) is identified in 757 of 5394 (14%) analyzed female tumors in the TCGA.Citation107 In female cells, one of the two X chromosomes is silenced, suggesting minimal impact from the loss of the silenced copy. However, not much is known about the role of X chromosome in tumorigenesis. Overall, with some potential exceptions regarding the sex chromosomes in specific cancer types, passenger aneuploidies with neither negative nor positive effects on cancer fitness are likely rare.
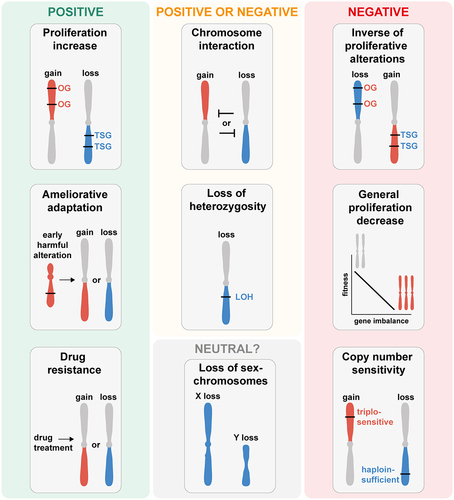
In sum, there are likely many positive and negative selection forces acting simultaneously for any individual aneuploid chromosome (). Whether an aneuploid chromosome is beneficial or not for tumor growth depends on the cumulation of these forces. Depending on the context influencing the selection forces, aneuploidies that can be beneficial in a certain tumor type or cell line can be disadvantageous in another. This becomes even more complex in the context of additional aneuploid chromosomes, mutations, and external selective forces.
Figure 3. Selection forces underlying the formation of aneuploidy patterns in cancer and human cell lines. Illustration of selection forces that shape the formation of aneuploidy patterns based on the observations from cancers, human cell lines, and model systems. Depending on environmental factors, cell type, and chromosome identity, aneuploidy patterns can be driven by positive, negative, or neutral selection forces. Positive selection forces underlying the copy number changes of a chromosome arm include: drivers of increased proliferation (upper left panel), compensating for harmful alterations (middle left panel) and conferring drug resistance (lower left panel). Selection forces that could be positive or negative depending on the context include: copy number loss resulting in loss of hetero-zygosity (LOH) and genetic interactions between aneuploid chromosomes (upper middle panels). Negative selection forces against chromosome copy number changes include: antagonization of proliferative alterations (upper right panel), the general proliferation decrease resulting from the imbalanced expression of many genes (middle right panel), and triplosensitive or haploinsufficient genes (lower right panel). Neutral selection of aneuploidy patterns is likely very rare and might only occur for aneuploidy of the sex chromosomes in specific cancer or cell types (lower middle panel). Chromosome arm gain is depicted in red, chromosome arm loss in blue.
5. How many genes drive aneuploidy patterns?
One of the key questions remaining for the selection of aneuploid patterns relates to how many genes are involved in contributing to their selection. Are these chromosomes being gained or lost due primarily to a single gene or the combinatorial impact of expression changes of many genes? This question gets to the heart of why aneuploidy may be so frequently observed in cancer as compared to other forms of mutation. There is some intriguing correlative evidence suggesting multiple genes may contribute to aneuploid chromosome selection. For example, the four most frequently mutated tumor suppressor genes in ccRCC (VHL, PBRM1, SETD2 and BAP1) are all located on chromosome arm 3p, and 3p loss is the most common aneuploidy in this cancer type.Citation109 However, determining the number of genes that contribute to the formation of aneuploidy patterns has been challenging and there is currently a dearth of direct evidence for any particular genes being necessary or sufficient.
One source of examples of aneuploidy patterns comes from in vitro evolution of model systems, where specific genes have been implicated in aneuploid chromosome selection to a variety of stresses and mutations. In some cases, these genes have been deleted from the aneuploid chromosomes to determine the degree to which they contribute to the observed phenotypes. The model system where such dependencies have been primarily studied is budding yeast. There have been a few cases where a single gene has been found to be necessary and sufficient to fully recapitulate an aneuploidy-associated phenotype.Citation80,Citation110 However, the majority of cases have been shown to involve at least two genes.Citation79 Using a mouse model with a partial chromosome deletion syntenic to human chromosome 17p13.1 encompassing the TP53 gene, Liu and colleagues showed that the advantageous effect resulting from the deletion of human chromosome 17p on cancer development is a consequence of both the absence of TP53 and the reduced levels of linked tumor suppressor genes working in conjunction, i.e. Eif5a and Alox15b.Citation111
There have been a few cases where this question has been directly addressed in human cells. In a model for Ewing sarcoma that leads to the frequent gain of chromosome 8, extra copies of the genes for MYC and the cohesin subunit RAD21 were shown to contribute to an increase in cell proliferation when combined. However, together they were still not sufficient to fully recapitulate the aneuploid phenotype, suggesting that additional genes on chromosome 8 contribute to the phenotype.Citation50 In a recent study of cancer cell lines with 1q gain, it was shown that deletion of the extra copy of MDM4 gene reduced proliferation, but not to the same extent as removing the extra copy of the entire arm.Citation11 In human liver cancer cell lines, the loss of chromosome arm 8p leads to increased migration and invasion. The overexpression of individual candidate genes located on chromosome 8p within the context of chromosome 8p deletion only partially reversed the migratory phenotype. However, the simultaneous overexpression of a combination of four genes led to a migratory capacity comparable to their overexpression in euploid wildtype cells.Citation12 These findings suggest that multiple genes contribute to the selection of aneuploid chromosomes in cancer.
One possibility for how to identify if multiple genes drive a phenotype of an aneuploidy is through engineering segmental SCNAs. This has been done for the chromosome arm deletions of 6p and 13q to determine regions responsible for drug resistance.Citation8 This technique could be used to uncover regions on other aneuploid chromosomes that drive aneuploidy phenotypes, helping to exactly determine how many chromosomal regions contribute to aneuploid chromosome selection. For now, the identification of the number of genes typically involved in aneuploid chromosome selection and the extent to which they act additively or synergistically remains to be determined.
6. Influence of whole genome duplication on aneuploidy patterns
After aneuploidy, whole genome duplication (WGD) is the second most common chromosome-level genome alteration in cancer cells. WGD occurs in approximately one third of human tumorsCitation112,Citation113,Citation114 and is associated with worse prognosis and survival.Citation112 Generally, WGD is correlated with a higher degree of aneuploidy.Citation6,Citation112 Most WGD tumors are near triploid which indicates that WGD might be more permissive for chromosome loss.Citation112,Citation115 Whether chromosome losses occur before or after WGD seems to differ between cancer types and type of analyses. For instance, researchers have identified that 70% of acquired heterozygous losses arose after WGD.Citation112 In contrast, Carter and colleagues identified that chromosome arm copy number changes occur mostly prior to genome doubling.Citation116 This is also seen in basal-like breast carcinoma, in which WGD occurred after multiple chromosome losses.Citation117 Additionally, WGD tumors have a more diverse spectrum of aneuploidies indicating greater tolerance to various chromosomes being aneuploid.Citation113 The better tolerance might be partially explained by the smaller relative effect of one copy number change in tetraploid cells compared to diploid cells. The higher ploidy decreases the impact of expression imbalances from gaining one chromosome copy from 1/3 to 1/5 and losing one copy from 1/2 to 1/4. Associated with reduced effect of chromosome loss, polyploidy was proposed to protect from LOH.Citation118 A recent study suggests that WGD influences complex aneuploidy patterns in tumors. In yeast and in human tumors, complex aneuploidy patterns are affected by initial ploidy.Citation80,Citation113 In cancer, the frequent co-occurrence of certain chromosome arms can change to mutual exclusivity and vice versa depending on the WGD status.Citation113 However, in general, tumor type-specific aneuploidy patterns are independent of WGD status.Citation113 Collectively, these analyses suggest minimal influence of WGD on pan-cancer and cancer type-specific aneuploidy patterns but identified a role of WGD in the formation of complex aneuploidy patterns.
7. Relevance of aneuploidy patterns for cancer treatment
The absence or presence of aneuploidy in cells has been utilized as a fundamental differentiator between transformed and healthy cellular states in various tumor types, rendering aneuploidy an attractive and selectively targetable hallmark of tumors.Citation119 This selective targeting approach holds promise for therapies that can exert their effects predominantly on cancer cells while minimizing adverse impacts on healthy counterparts.
One approach being explored is targeting the specific vulnerabilities associated with aneuploid chromosomes in general, such as proteotoxic stress,Citation120,Citation121 DNA damage response,Citation122 or sphingolipid synthesis.Citation123 In addition, therapies based on specific frequent aneuploidies may hold promise as personalized therapeutic strategies. One potential benefit of targeting the specific aneuploidy patterns of a given tumor would be greater specificity for elimination of the specific tumor cells compared to general chemotherapeutics. This approach seems especially encouraging and supported by the recent identification that cancer cell lines are “addicted” to their aneuploidies.Citation11
However, there are currently many challenges associated with developing treatments targeting aneuploid chromosomes. Firstly, we do not know the genetic basis that drives the selection of most aneuploid chromosomes in tumors, making it impossible to design specific strategies for treatments based on their specific contributions to cancer. Second, we know little about which of the non-driver genes on the aneuploid chromosome might underlie the chromosome-specific negative proliferation effects. These other dosage sensitive genes could be targeted with treatments exploiting chromosome-specific vulnerabilities.Citation124 Recently, Girish and colleagues discovered that the frequent gain of chromosome 1q in breast cancer also changes the protein abundance of UCK2 making cells with a chromosome 1q gain more sensitive to certain drugs.Citation11 Gene imbalances from aneuploidy could also be exploited for synthetic lethal interactions. A recent study identified deletion of the reactive oxygen sensing enzyme NUDT17 as synthetic lethal with an engineered chromosome 8p deletion cell line.Citation12 Finally, one limitation for targeting aneuploidy patterns instead of gene mutations could be the relatively easy reversal of the targeted aneuploid chromosome to euploid levels in cancer cells. Changing the copy number of one chromosome can be achieved through a mis-segregation event during cell division which is very likely to occur in cancer cells. In chromosomally unstable colorectal cancer cell lines, it has been observed that in one out of five cell divisions, chromosomes become mis-segregated.Citation125–127, By comparison, reverting a gene mutation will occur much less frequently. Therefore, targeting aneuploidy patterns in tumors might only be realistic in the context of combinatorial treatments that additionally target other vulnerabilities.
8. Concluding remarks and future perspectives
In summary, the high prevalence and specificity of different types of aneuploidy patterns underscores their context-dependent relevance for tumorigenesis and indicates positive selection. There is growing evidence for aneuploidy patterns playing cancer type-specific roles in tumor initiation, progression, metastasis formation, immune evasion and resistance to therapeutic treatment. Despite being pervasive in human tumors, the genetic basis of aneuploidy pattern selection largely remains to be uncovered. The selection of aneuploidies likely depends on various positive and negative forces which amount to either a net beneficial or detrimental consequence on tumor cell growth. While there is some evidence of different driver genes shaping aneuploidy patterns in cancer, further research is necessary to identify the most impactful driver genes. In addition, cancer type-specific genetic vulnerabilities associated with certain aneuploidy patterns are just beginning to be investigated.
Abbreviations
| BLCA | = | BLadder Urothelial CArcinoma |
| BRCA | = | BReast Invasive CArcinoma |
| ccRCC | = | clear cell Renal Cell Carcinoma |
| CIN | = | Chromosome INstability |
| COAD | = | COlon ADenocarcinoma |
| COADREAD | = | COlon ADenocarcinoma (COAD) and REctum ADenocarcinoma (READ) |
| COSMIC | = | Catalogue Of Somatic Mutations In Cancer |
| FHWT | = | Favorable Histology Wilms Tumor |
| GBM | = | GlioBlastoma Multiforme |
| GBMLGG | = | GlioBlastoma Multiforme and Brain Lower Grade Glioma |
| HCC | = | HepatoCellular Carcinoma |
| HNC | = | Head and Neck Cancer |
| KIRP | = | KIdney Renal Papillary Cell Carcinoma |
| LOH | = | Loss Of Heterozygosity |
| LOX | = | Loss Of X chromosome |
| LOY | = | Loss Of Y chromosome |
| MESO | = | MESOthelioma |
| NSCLC | = | Non-Small Cell Lung Cancer |
| OG | = | OncoGene |
| PANSCC | = | PAN-Squamous Cell Carcinoma |
| SCNAs | = | Somatic Copy Number Alterations |
| SKCM | = | SKin Cutaneous Melanoma |
| SNV | = | Single Nucleotide Variants |
| TCGA | = | The Cancer Genome Atlas |
| TSG | = | Tumor Suppressor Gene |
| UVM | = | UVeal Melanoma |
| WGD | = | Whole Genome Duplication |
Author contributions
T.C.K. and C.S.C. together conceptualized and wrote the manuscript.
Acknowledgments
We would like to thank Dea Slade and Alex Dammermann for critical reading of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Hardy P, Zacharias H. Reappraisal of the Hansemann–Boveri hypothesis on the origin of tumors. Cell Biol Int. 2005;29(12):983–15. doi:10.1016/j.cellbi.2005.10.001.
- Baudis M. Genomic imbalances in 5918 malignant epithelial tumors: An explorative meta-analysis of chromosomal CGH data. BMC Cancer. 2007;7(1):226. doi:10.1186/1471-2407-7-226.
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M. et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463(7283):899–905. doi:10.1038/nature08822.
- Mitelman F, Johansson B, Mertens F. Mitelman Database of Chromosome Aberrations and Gene Fusions in Cancer. 2023 [Accessed 2023 Aug 23]. https://mitelmandatabase.isb-cgc.org.
- Aaltonen LA, Abascal F, Abeshouse A, Aburatani H, Adams DJ, Agrawal N, Ahn KS, Ahn S-M, Aikata H, Akbani R. et al. Pan-cancer analysis of whole genomes. Nature. 2020;578(7793):82–93. doi:10.1038/s41586-020-1969-6.
- Taylor AM, Shih J, Ha G, Gao GF, Zhang X, Berger AC, Schumacher SE, Wang C, Hu H, Liu J. et al. Genomic and Functional Approaches to Understanding Cancer Aneuploidy. Cancer Cell. 2018;33(4):676–689.e3. doi:10.1016/j.ccell.2018.03.007.
- Weaver BA, Cleveland DW. Does aneuploidy cause cancer? Curr Opin Cell Biol. 2006;18(6):658–667. doi:10.1016/j.ceb.2006.10.002.
- Adell MAY, Klockner TC, Höfler R, Wallner L, Schmid J, Markovic A, Martyniak A, Campbell CS. Adaptation to spindle assembly checkpoint inhibition through the selection of specific aneuploidies. Genes Dev. 2023;37(5–6):171–190. doi:10.1101/gad.350182.122.
- Bosco N, Goldberg A, Zhao X, Mays JC, Cheng P, Johnson AF, Bianchi JJ, Toscani C, Di Tommaso E, Katsnelson L. et al. KaryoCreate: A CRISPR-based technology to study chromosome-specific aneuploidy by targeting human centromeres. Cell. 2023;186(9):S1985–2001.e19. doi:10.1016/j.cell.2023.03.029.
- Chunduri NK, Menges P, Zhang X, Wieland A, Gotsmann VL, Mardin BR, Buccitelli C, Korbel JO, Willmund F, Kschischo M. et al. Systems approaches identify the consequences of monosomy in somatic human cells. Nat Commun. 2021;12(1):5576. doi:10.1038/s41467-021-25288-x.
- Girish V, Lakhani AA, Thompson SL, Scaduto CM, Brown LM, Hagenson RA, Sausville EL, Mendelson BE, Kandikuppa PK, Lukow DA. et al. Oncogene-like addiction to aneuploidy in human cancers. Science. 2023;381(6660):eadg4521. doi:10.1126/science.adg4521.
- Huth T, Dreher EC, Lemke S, Fritzsche S, Sugiyanto RN, Castven D, Ibberson D, Sticht C, Eiteneuer E, Jauch A. et al. Chromosome 8p engineering reveals increased metastatic potential targetable by patient-specific synthetic lethality in liver cancer. Sci Adv. 2023;9(51):eadh1442. doi:10.1126/sciadv.adh1442.
- Shih J, Sarmashghi S, Zhakula-Kostadinova N, Zhang S, Georgis Y, Hoyt SH, Cuoco MS, Gao GF, Spurr LF, Berger AC. et al. Cancer aneuploidies are shaped primarily by effects on tumour fitness. Nature. 2023;619(7971):793–800. doi:10.1038/s41586-023-06266-3.
- Chunduri NK, Storchová Z. The diverse consequences of aneuploidy. Nat Cell Biol. 2019;21(1):54–62. doi:10.1038/s41556-018-0243-8.
- Lakhani AA, Thompson SL, Sheltzer JM. Aneuploidy in human cancer: New tools and perspectives. Trends Genet. 2023;39(12):S968–980. doi:10.1016/j.tig.2023.09.002.
- Thompson SL, Bakhoum SF, Compton DA. Mechanisms of Chromosomal Instability. Curr Biol. 2010;20(6):R285–R295. doi:10.1016/j.cub.2010.01.034.
- Zhu J, Tsai H-J, Gordon MR, Li R. Cellular Stress Associated with Aneuploidy. Dev Cell. 2018;44(4):420–431. doi:10.1016/j.devcel.2018.02.002.
- Donehower LA, Soussi T, Korkut A, Liu Y, Schultz A, Cardenas M, Li X, Babur O, Hsu T-K, Lichtarge O. et al. Integrated Analysis of TP53 Gene and Pathway Alterations in the Cancer Genome Atlas. Cell Rep. 2019;28(5):1370–1384.e5. doi:10.1016/j.celrep.2019.07.001.
- Ghandi M, Huang FW, Jané-Valbuena J, Kryukov GV, Lo CC, McDonald ER, Barretina J, Gelfand ET, Bielski CM, Li H. et al. Next-generation characterization of the Cancer Cell Line Encyclopedia. Nature. 2019;569(7757):503–508. doi:10.1038/s41586-019-1186-3.
- Zehir A, Benayed R, Shah RH, Syed A, Middha S, Kim HR, Srinivasan P, Gao J, Chakravarty D, Devlin SM. et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. doi:10.1038/nm.4333.
- Nicholson JM, Cimini D. Cancer Karyotypes: Survival of the Fittest. Front Oncol. 2013;3:3. doi:10.3389/fonc.2013.00148.
- Ried T, Hu Y, Difilippantonio MJ, Ghadimi BM, Grade M, Camps J. The consequences of chromosomal aneuploidy on the transcriptome of cancer cells. Biochim Biophys Acta (BBA) - Gene Regulatory Mechanisms. 2012;1819(7):784–793. doi:10.1016/j.bbagrm.2012.02.020.
- The Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi:10.1038/nature11252.
- Crespo I, Vital AL, Nieto AB, Rebelo O, Tão H, Lopes MC, Oliveira CR, French PJ, Orfao A, Tabernero MD. Detailed Characterization of Alterations of Chromosomes 7, 9, and 10 in Glioblastomas as Assessed by Single-Nucleotide Polymorphism Arrays. J Mol Diagn. 2011;13(6):634–647. doi:10.1016/j.jmoldx.2011.06.003.
- Buerger H, Otterbach F, Simon R, Poremba C, Diallo R, Decker T, Riethdorf L, Brinkschmidt C, Dockhorn-Dworniczak B, Boecker W. Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J Pathol. 1999;187(4):396–402. doi:10.1002/(SICI)1096-9896(199903)187:4<396:AID-PATH286>3.0.CO;2-L.
- Ciriello G, Miller ML, Aksoy BA, Senbabaoglu Y, Schultz N, Sander C. Emerging landscape of oncogenic signatures across human cancers. Nat Genet. 2013;45(10):1127–1133. doi:10.1038/ng.2762.
- Watkins TB. Pervasive chromosomal instability and karyotype order in tumour evolution. Nature. 2020;587(7832):126–132. doi:10.1038/s41586-020-2698-6.
- Gerstung M, Jolly C, Leshchiner I, Dentro SC, Gonzalez S, Rosebrock D, Mitchell TJ, Rubanova Y, Anur P, Yu K. et al. The evolutionary history of 2,658 cancers. Nature. 2020;578(7793):122–128. doi:10.1038/s41586-019-1907-7.
- Douville C, Moinova HR, Thota PN, Shaheen NJ, Iyer PG, Canto MI, Wang JS, Dumot JA, Faulx A, Kinzler KW. et al. Massively Parallel Sequencing of Esophageal Brushings Enables an Aneuploidy-Based Classification of Patients with Barrett’s Esophagus. Gastroenterology. 2021;160(6):2043–2054.e2. doi:10.1053/j.gastro.2021.01.209.
- Abe I, Suzuki K, Kimura Y, Tamaki S, Endo Y, Ichida K, Muto Y, Watanabe F, Saito M, Konishi F. et al. Enhancement of DNA hypomethylation alterations by gastric and bile acids promotes chromosomal instability in Barrett’s epithelial cell line. Sci Rep. 2022;12(1):20710. doi:10.1038/s41598-022-25279-y.
- Mitchell TJ, Turajlic S, Rowan A, Nicol D, Farmery JHR, O’Brien T, Martincorena I, Tarpey P, Angelopoulos N, Yates LR. et al. Timing the Landmark Events in the Evolution of Clear Cell Renal Cell Cancer: TRACERx Renal. Cell. 2018;173(3):611–623.e17. doi:10.1016/j.cell.2018.02.020.
- Körber V, Yang J, Barah P, Wu Y, Stichel D, Gu Z, Fletcher MNC, Jones D, Hentschel B, Lamszus K. et al. Evolutionary Trajectories of IDHWT Glioblastomas Reveal a Common Path of Early Tumorigenesis Instigated Years ahead of Initial Diagnosis. Cancer Cell. 2019;35(4):692–704.e12. doi:10.1016/j.ccell.2019.02.007.
- Hasle H, Clemmensen IH, Mikkelsen M. Risks of leukaemia and solid tumours in individuals with Down’s syndrome. Lancet (Engl). 2000;355(9199):165–169. doi:10.1016/S0140-6736(99)05264-2.
- Hasle H, Friedman JM, Olsen JH, Rasmussen SA. Low risk of solid tumors in persons with Down syndrome. Genet Med. 2016;18(11):1151–1157. doi:10.1038/gim.2016.23.
- Duijf PHG, Schultz N, Benezra R. Cancer cells preferentially lose small chromosomes. Int J Cancer. 2013;132(10):2316–2326. doi:10.1002/ijc.27924.
- Mitelman F, Heim S, Mandahl N. Trisomy 21 in neoplastic cells. Am J Med Genet. 2005;37(S7):262–266. doi:10.1002/ajmg.1320370752.
- Williams BR, Prabhu VR, Hunter KE, Glazier CM, Whittaker CA, Housman DE, Amon A. Aneuploidy Affects Proliferation and Spontaneous Immortalization in Mammalian Cells. Science. 2008;322(5902):703–709. doi:10.1126/science.1160058.
- Foijer F, Xie SZ, Simon JE, Bakker PL, Conte N, Davis SH, Kregel E, Jonkers J, Bradley A, Sorger PK. Chromosome instability induced by Mps1 and p53 mutation generates aggressive lymphomas exhibiting aneuploidy-induced stress. Proc Natl Acad Sci USA. 2014;111(37):13427–13432. doi:10.1073/pnas.1400892111.
- Hoevenaar WHM, Janssen A, Quirindongo AI, Ma H, Klaasen SJ, Teixeira A, Van Gerwen B, Lansu N, Morsink FHM, Offerhaus GJA. et al. Degree and site of chromosomal instability define its oncogenic potential. Nat Commun. 2020;11(1):1501. doi:10.1038/s41467-020-15279-9.
- Jeganathan K, Malureanu L, Baker DJ, Abraham SC, Van Deursen JM. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumorigenesis. J Cell Biol. 2007;179(2):255–267. doi:10.1083/jcb.200706015.
- Levine MS, Bakker B, Boeckx B, Moyett J, Lu J, Vitre B, Spierings DC, Lansdorp PM, Cleveland DW, Lambrechts D. et al. Centrosome Amplification is Sufficient to Promote Spontaneous Tumorigenesis in Mammals. Dev Cell. 2017;40(3):313–322.e5. doi:10.1016/j.devcel.2016.12.022.
- Li M, Fang X, Wei Z, York JP, Zhang P. Loss of spindle assembly checkpoint–mediated inhibition of Cdc20 promotes tumorigenesis in mice. J Cell Biol. 2009;185(6):983–994. doi:10.1083/jcb.200904020.
- Weaver BAA, Silk AD, Montagna C, Verdier-Pinard P, Cleveland DW. Aneuploidy Acts Both Oncogenically and as a Tumor Suppressor. Cancer Cell. 2007;11(1):25–36. doi:10.1016/j.ccr.2006.12.003.
- Trakala M, Aggarwal M, Sniffen C, Zasadil L, Carroll A, Ma D, Su XA, Wangsa D, Meyer A, Sieben CJ. et al. Clonal selection of stable aneuploidies in progenitor cells drives high-prevalence tumorigenesis. Genes Dev. 2021;35(15–16):1079–1092. doi:10.1101/gad.348341.121.
- Santaguida S, Richardson A, Iyer DR, M’Saad O, Zasadil L, Knouse KA, Wong YL, Rhind N, Desai A, Amon A. Chromosome mis-segregation generates cell-cycle-arrested cells with complex karyotypes that are eliminated by the Immune System. Dev Cell. 2017;41(6):638–651.e5. doi:10.1016/j.devcel.2017.05.022.
- Sheltzer JM, Ko JH, Replogle JM, Habibe Burgos NC, Chung ES, Meehl CM, Sayles NM, Passerini V, Storchova Z, Amon A. Single-chromosome gains commonly function as tumor suppressors. Cancer Cell. 2017;31(2):240–255. doi:10.1016/j.ccell.2016.12.004.
- Stopsack KH, Whittaker CA, Gerke TA, Loda M, Kantoff PW, Mucci LA, Amon A. Aneuploidy drives lethal progression in prostate cancer. Proc Natl Acad Sci USA. 2019;116(23):11390–11395. doi:10.1073/pnas.1902645116.
- Vasudevan A, Baruah PS, Smith JC, Wang Z, Sayles NM, Andrews P, Kendall J, Leu J, Chunduri NK, Levy D. et al. Single-chromosomal gains can function as metastasis suppressors and promoters in colon cancer. Dev Cell. 2020;52(4):413–428.e6. doi:10.1016/j.devcel.2020.01.034.
- Shukla A, Nguyen THM, Moka SB, Ellis JJ, Grady JP, Oey H, Cristino AS, Khanna KK, Kroese DP, Krause L. et al. Chromosome arm aneuploidies shape tumour evolution and drug response. Nat Commun. 2020;11(1):449. doi:10.1038/s41467-020-14286-0.
- Su XA, Ma D, Parsons JV, Replogle JM, Amatruda JF, Whittaker CA, Stegmaier K, Amon A. RAD21 is a driver of chromosome 8 gain in Ewing sarcoma to mitigate replication stress. Genes Dev. 2021;35(7–8):556–572. doi:10.1101/gad.345454.120.
- Davoli T, Uno H, Wooten EC, Elledge SJ. Tumor aneuploidy correlates with markers of immune evasion and with reduced response to immunotherapy. Science. 2017;355(6322):eaaf8399. doi:10.1126/science.aaf8399.
- Chang T-G, Cao Y, Shulman ED, Ben-David U, Schäffer AA, Ruppin E. Optimizing cancer immunotherapy response prediction by tumor aneuploidy score and fraction of copy number alterations. NPJ Precis Oncol. 2023;7(1):54. doi:10.1038/s41698-023-00408-6.
- Spurr LF, Weichselbaum RR, Pitroda SP. Tumor aneuploidy predicts survival following immunotherapy across multiple cancers. Nat Genet. 2022;54(12):1782–1785. doi:10.1038/s41588-022-01235-4.
- William WN, Zhao X, Bianchi JJ, Lin HY, Cheng P, Lee JJ, Carter H, Alexandrov LB, Abraham JP, Spetzler DB. et al. Immune evasion in HPV − head and neck precancer–cancer transition is driven by an aneuploid switch involving chromosome 9p loss. Proc Natl Acad Sci USA. 2021;118(19):e2022655118. doi:10.1073/pnas.2022655118.
- Bakhoum SF, Ngo B, Laughney AM, Cavallo J-A, Murphy CJ, Ly P, Shah P, Sriram RK, Watkins TBK, Taunk NK. et al. Chromosomal instability drives metastasis through a cytosolic DNA response. Nature. 2018;553(7689):467–472. doi:10.1038/nature25432.
- Hong C, Schubert M, Tijhuis AE, Requesens M, Roorda M, van den Brink A, Ruiz LA, Bakker PL, van der Sluis T, Pieters W. et al. cGAS-STING drives the IL-6-dependent survival of chromosomally instable cancers. Nature. 2022;607(7918):366–373. doi:10.1038/s41586-022-04847-2.
- Li J, Hubisz MJ, Earlie EM, Duran MA, Hong C, Varela AA, Lettera E, Deyell M, Tavora B, Havel JJ. et al. Non-cell-autonomous cancer progression from chromosomal instability. Nature. 2023;620(7976):1080–1088. doi:10.1038/s41586-023-06464-z.
- Gadd S, Huff V, Skol AD, Renfro LA, Fernandez CV, Mullen EA, Jones CD, Hoadley KA, Yap KL, Ramirez NC. et al. Genetic changes associated with relapse in favorable histology Wilms tumor: A Children’s Oncology group AREN03B2 study. Cell Rep Med. 2022;3(6):100644. doi:10.1016/j.xcrm.2022.100644.
- Ippolito MR, Martis V, Martin S, Tijhuis AE, Hong C, Wardenaar R, Dumont M, Zerbib J, Spierings DCJ, Fachinetti D. et al. Gene copy-number changes and chromosomal instability induced by aneuploidy confer resistance to chemotherapy. Dev Cell. 2021;56(17):2440–2454.e6. doi:10.1016/j.devcel.2021.07.006.
- Lukow DA, Sausville EL, Suri P, Chunduri NK, Wieland A, Leu J, Smith JC, Girish V, Kumar AA, Kendall J. et al. Chromosomal instability accelerates the evolution of resistance to anti-cancer therapies. Dev Cell. 2021;56(17):2427–2439.e4. doi:10.1016/j.devcel.2021.07.009.
- Martínez-Jiménez F, Movasati A, Brunner SR, Nguyen L, Priestley P, Cuppen E, Van Hoeck A. Pan-cancer whole-genome comparison of primary and metastatic solid tumours. Nature. 2023;618(7964):333–341. doi:10.1038/s41586-023-06054-z.
- Nguyen B, Fong C, Luthra A, Smith SA, DiNatale RG, Nandakumar S, Walch H, Chatila WK, Madupuri R, Kundra R. et al. Genomic characterization of metastatic patterns from prospective clinical sequencing of 25,000 patients. Cell. 2022;185(3):563–575.e11. doi:10.1016/j.cell.2022.01.003.
- Turajlic S, Xu H, Litchfield K, Rowan A, Horswell S, Chambers T, O’Brien T, Lopez JI, Watkins TBK, Nicol D. et al. Deterministic evolutionary trajectories influence primary tumor growth: TRACERx Renal. Cell. 2018;173(3):595–610.e11. doi:10.1016/j.cell.2018.03.043.
- Perelli L, Carbone F, Zhang L, Huang JK, Le C, Khan H, Citron F, Del Poggetto E, Gutschner T, Tomihara H. et al. Interferon signaling promotes tolerance to chromosomal instability during metastatic evolution in renal cancer. Nat Cancer. 2023;4(7):984–1000. doi:10.1038/s43018-023-00584-1.
- Holland AJ, Cleveland DW. Losing balance: The origin and impact of aneuploidy in cancer: ‘Exploring aneuploidy: the significance of chromosomal imbalance’ review series. EMBO Rep. 2012;13(6):501–514. doi:10.1038/embor.2012.55.
- Klaasen SJ, Truong MA, Van Jaarsveld RH, Koprivec I, Štimac V, De Vries SG, Risteski P, Kodba S, Vukušić K, De Luca KL. et al. Nuclear chromosome locations dictate segregation error frequencies. Nature. 2022;607(7919):604–609. doi:10.1038/s41586-022-04938-0.
- Worrall JT, Tamura N, Mazzagatti A, Shaikh N, van Lingen T, Bakker B, Spierings DCJ, Vladimirou E, Foijer F, McClelland SE. Non-random Mis-segregation of Human Chromosomes. Cell Rep. 2018;23(11):3366–3380. doi:10.1016/j.celrep.2018.05.047.
- Dephoure N, Hwang S, O’Sullivan C, Dodgson SE, Gygi SP, Amon A, Torres EM. Quantitative proteomic analysis reveals posttranslational responses to aneuploidy in yeast. eLife. 2014;3:e03023. doi:10.7554/eLife.03023.
- Stingele S, Stoehr G, Peplowska K, Cox J, Mann M, Storchova Z. Global analysis of genome, transcriptome and proteome reveals the response to aneuploidy in human cells. Mol Syst Biol. 2012;8(1):608. doi:10.1038/msb.2012.40.
- Torres EM, Springer M, Amon A. No current evidence for widespread dosage compensation in S. cerevisiae. eLife. 2016;5:e10996. doi:10.7554/eLife.10996.
- Upender MB, Habermann JK, McShane LM, Korn EL, Barrett JC, Difilippantonio MJ, Ried T. Chromosome Transfer Induced Aneuploidy Results in Complex Dysregulation of the Cellular Transcriptome in Immortalized and Cancer Cells. Cancer Res. 2004;64(19):6941–6949. doi:10.1158/0008-5472.CAN-04-0474.
- Brennan CM, Vaites LP, Wells JN, Santaguida S, Paulo JA, Storchova Z, Harper JW, Marsh JA, Amon A. Protein aggregation mediates stoichiometry of protein complexes in aneuploid cells. Genes Dev. 2019;33(15–16):1031–1047. doi:10.1101/gad.327494.119.
- Schukken KM, Sheltzer JM. Extensive protein dosage compensation in aneuploid human cancers. Genome Res. 2022;32(7):1254–1270. doi:10.1101/gr.276378.121.
- Muenzner J. Natural proteome diversity links aneuploidy tolerance to protein turnover. Nature. 2024;630(8015):149–157. doi:10.1038/s41586-024-07442-9.
- Davoli T, Xu AW, Mengwasser KE, Sack LM, Yoon JC, Park PJ, Elledge SJ. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell. 2013;155(4):948–962. doi:10.1016/j.cell.2013.10.011.
- Sack LM, Davoli T, Li MZ, Li Y, Xu Q, Naxerova K, Wooten EC, Bernardi RJ, Martin TD, Chen T. et al. Profound tissue specificity in proliferation control underlies cancer drivers and aneuploidy patterns. Cell. 2018;173(2):499–514.e23. doi:10.1016/j.cell.2018.02.037.
- Selmecki A, Forche A, Berman J. Aneuploidy and isochromosome formation in drug-resistant Candida albicans. Sci (New Y). 2006;313(5785):367–370. doi:10.1126/science.1128242.
- Selmecki A, Gerami‐Nejad M, Paulson C, Forche A, Berman J. An isochromosome confers drug resistance in vivo by amplification of two genes, ERG11 and TAC1. Mol Microbiol. 2008;68(3):624–641. doi:10.1111/j.1365-2958.2008.06176.x.
- Tsai H-J, Nelliat A. A double-edged sword: aneuploidy is a prevalent strategy in fungal adaptation. Genes. 2019;10(10):787. doi:10.3390/genes10100787.
- Ravichandran MC, Fink S, Clarke MN, Hofer FC, Campbell CS. Genetic interactions between specific chromosome copy number alterations dictate complex aneuploidy patterns. Genes Dev. 2018;32(23–24):1485–1498. doi:10.1101/gad.319400.118.
- Barriga FM, Tsanov KM, Ho Y-J, Sohail N, Zhang A, Baslan T, Wuest AN, Del Priore I, Meškauskaitė B, Livshits G. et al. MACHETE identifies interferon-encompassing chromosome 9p21.3 deletions as mediators of immune evasion and metastasis. Nat Cancer. 2022;3(11):1367–1385. doi:10.1038/s43018-022-00443-5.
- Watson EV, Lee J-K, Gulhan DC, Melloni GEM, Venev SV, Magesh RY, Frederick A, Chiba K, Wooten EC, Naxerova K. et al. Chromosome evolution screens recapitulate tissue-specific tumor aneuploidy patterns. Nat Genet. 2024;56(5):900–912. doi:10.1038/s41588-024-01665-2.
- Gialesaki S, Bräuer-Hartmann D, Issa H, Bhayadia R, Alejo-Valle O, Verboon L, Schmell A-L, Laszig S, Regényi E, Schuschel K. et al. RUNX1 isoform disequilibrium promotes the development of trisomy 21–associated myeloid leukemia. Blood. 2023;141(10):1105–1118. doi:10.1182/blood.2022017619.
- Laurent AP, Kotecha RS, Malinge S. Gain of chromosome 21 in hematological malignancies: Lessons from studying leukemia in children with Down syndrome. Leukemia. 2020;34(8):1984–1999. doi:10.1038/s41375-020-0854-5.
- Bakhoum SF, Landau DA. Cancer Evolution: No Room for Negative Selection. Cell. 2017;171(5):987–989. doi:10.1016/j.cell.2017.10.039.
- Martincorena I, Raine KM, Gerstung M, Dawson KJ, Haase K, Van Loo P, Davies H, Stratton MR, Campbell PJ. Universal Patterns of Selection in Cancer and Somatic Tissues. Cell. 2017;171(5):1029–1041.e21. doi:10.1016/j.cell.2017.09.042.
- Torres EM, Sokolsky T, Tucker CM, Chan LY, Boselli M, Dunham MJ, Amon A. Effects of aneuploidy on cellular physiology and cell division in haploid yeast. Science. 2007;317(5840):916–924. doi:10.1126/science.1142210.
- Beach RR, Ricci-Tam C, Brennan CM, Moomau CA, Hsu P-H, Hua B, Silberman RE, Springer M, Amon A. Aneuploidy Causes Non-genetic Individuality. Cell. 2017;169(2):229–242.e21. doi:10.1016/j.cell.2017.03.021.
- Hintzen DC, Soto M, Schubert M, Bakker B, Spierings DCJ, Szuhai K, Lansdorp PM, Kluin RJC, Foijer F, Medema RH. et al. The impact of monosomies, trisomies and segmental aneuploidies on chromosomal stability. PLOS ONE. 2022;17(7):e0268579. doi:10.1371/journal.pone.0268579.
- Donnelly N, Passerini V, Dürrbaum M, Stingele S, Storchová Z. HSF1 deficiency and impaired HSP90‐dependent protein folding are hallmarks of aneuploid human cells. Embo J. 2014;33(20):2374–2387. doi:10.15252/embj.201488648.
- Oromendia AB, Dodgson SE, Amon A. Aneuploidy causes proteotoxic stress in yeast. Genes Dev. 2012;26(24):2696–2708. doi:10.1101/gad.207407.112.
- Passerini V, Ozeri-Galai E, de Pagter MS, Donnelly N, Schmalbrock S, Kloosterman WP, Kerem B, Storchová Z. The presence of extra chromosomes leads to genomic instability. Nat Commun. 2016;7(1):10754. doi:10.1038/ncomms10754.
- Santaguida S, Vasile E, White E, Amon A. Aneuploidy-induced cellular stresses limit autophagic degradation. Genes Dev. 2015;29(19):2010–2021. doi:10.1101/gad.269118.115.
- Sheltzer JM, Blank HM, Pfau SJ, Tange Y, George BM, Humpton TJ, Brito IL, Hiraoka Y, Niwa O, Amon A. Aneuploidy Drives Genomic Instability in Yeast. Science. 2011;333(6045):1026–1030. doi:10.1126/science.1206412.
- Knouse KA, Wu J, Whittaker CA, Amon A. Single cell sequencing reveals low levels of aneuploidy across mammalian tissues. Proc Natl Acad Sci USA. 2014;111(37):13409–13414. doi:10.1073/pnas.1415287111.
- Hassold T, Hunt P. To err (meiotically) is human: The genesis of human aneuploidy. Nat Rev Genet. 2001;2(4):280–291. doi:10.1038/35066065.
- The Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474(7353):609–615. doi:10.1038/nature10166.
- Morrill SA, Amon A. Why haploinsufficiency persists. Proceedings of the National Academy of Sciences of the United States of America; Vol. 10. 2019. p. 201900437. doi:10.1073/pnas.1900437116.
- Bonney ME, Moriya H, Amon A. Aneuploid proliferation defects in yeast are not driven by copy number changes of a few dosage-sensitive genes. Genes Dev. 2015;29(9):898–903. doi:10.1101/gad.261743.115.
- Collins RL, Glessner JT, Porcu E, Lepamets M, Brandon R, Lauricella C, Han L, Morley T, Niestroj L-M, Ulirsch J. et al. A cross-disorder dosage sensitivity map of the human genome. Cell. 2022;185(16):3041–3055.e25. doi:10.1016/j.cell.2022.06.036.
- Zhao H, Cui Y, Dupont J, Sun H, Hennighausen L, Yakar S. Overexpression of the tumor suppressor gene phosphatase and tensin homologue partially inhibits Wnt-1–Induced mammary Tumorigenesis. Cancer Res. 2005;65(15):6864–6873. doi:10.1158/0008-5472.CAN-05-0181.
- Valente P, Melchiori A, Paggi MG, Masiello L, Ribatti D, Santi L, Takahashi R, Albini A, Noonan DM. RB1 oncosuppressor gene over-expression inhibits tumor progression and induces melanogenesis in metastatic melanoma cells. Oncogene. 1996;13(6):1169–1178. PMID: 8808691.
- Ozery-Flato M, Linhart C, Trakhtenbrot L, Izraeli S, Shamir R. Large-scale analysis of chromosomal aberrations in cancer karyotypes reveals two distinct paths to aneuploidy. Genome Biol. 2011;12(6):R61. doi:10.1186/gb-2011-12-6-r61.
- Ciani Y, Fedrizzi T, Prandi D, Lorenzin F, Locallo A, Gasperini P, Franceschini GM, Benelli M, Elemento O, Fava LL. et al. Allele-specific genomic data elucidate the role of somatic gain and copy-number neutral loss of heterozygosity in cancer. Cell Syst. 2022;13(2):183–193.e7. doi:10.1016/j.cels.2021.10.001.
- Nichols CA, Gibson WJ, Brown MS, Kosmicki JA, Busanovich JP, Wei H, Urbanski LM, Curimjee N, Berger AC, Gao GF. et al. Loss of heterozygosity of essential genes represents a widespread class of potential cancer vulnerabilities. Nat Commun. 2020;11(1):2517. doi:10.1038/s41467-020-16399-y.
- Van Den Eynden J, Basu S, Larsson E, Gordenin DA. Somatic mutation patterns in hemizygous genomic regions unveil purifying selection during tumor evolution. PLOS Genet. 2016;12(12):e1006506. doi:10.1371/journal.pgen.1006506.
- Qi M, Pang J, Mitsiades I, Lane AA, Rheinbay E. Loss of chromosome Y in primary tumors. Cell. 2023;186(14):3125–3136.e11. doi:10.1016/j.cell.2023.06.006.
- Abdel-Hafiz HA, Schafer JM, Chen X, Xiao T, Gauntner TD, Li Z, Theodorescu D. Y chromosome loss in cancer drives growth by evasion of adaptive immunity. Nature. 2023;619(7970):624–631. doi:10.1038/s41586-023-06234-x.
- Hsieh JJ, Le VH, Oyama T, Ricketts CJ, Ho TH, Cheng EH. Chromosome 3p loss–orchestrated VHL, HIF, and epigenetic deregulation in clear cell renal cell carcinoma. J Clin Oncol. 2018;36(36):3533–3539. doi:10.1200/JCO.2018.79.2549.
- Pavelka N, Rancati G, Zhu J, Bradford WD, Saraf A, Florens L, Sanderson BW, Hattem GL, Li R. Aneuploidy confers quantitative proteome changes and phenotypic variation in budding yeast. Nature. 2010;468(7321):321–325. doi:10.1038/nature09529.
- Liu Y, Chen C, Xu Z, Scuoppo C, Rillahan CD, Gao J, Spitzer B, Bosbach B, Kastenhuber ER, Baslan T. et al. Deletions linked to TP53 loss drive cancer through p53-independent mechanisms. Nature. 2016;531(7595):471–475. doi:10.1038/nature17157.
- Bielski CM, Zehir A, Penson AV, Donoghue MTA, Chatila W, Armenia J, Chang MT, Schram AM, Jonsson P, Bandlamudi C. et al. Genome doubling shapes the evolution and prognosis of advanced cancers. Nat Genet. 2018;50(8):1189–1195. doi:10.1038/s41588-018-0165-1.
- Prasad K, Bloomfield M, Levi H, Keuper K, Bernhard SV, Baudoin NC, Leor G, Eliezer Y, Giam M, Wong CK. et al. Whole-genome duplication shapes the aneuploidy landscape of human cancers. Cancer Res. 2022;82(9):1736–1752. doi:10.1158/0008-5472.CAN-21-2065.
- Zack TI, Schumacher SE, Carter SL, Cherniack AD, Saksena G, Tabak B, Lawrence MS, Zhang C-Z, Wala J, Mermel CH. et al. Pan-cancer patterns of somatic copy number alteration. Nat Genet. 2013;45(10):1134–1140. doi:10.1038/ng.2760.
- Wangsa D, Quintanilla I, Torabi K, Vila‐Casadesús M, Ercilla A, Klus G, Yuce Z, Galofré C, Cuatrecasas M, José Lozano J. et al. Near‐tetraploid cancer cells show chromosome instability triggered by replication stress and exhibit enhanced invasiveness. The FASEB J. 2018;32(7):3502–3517. doi:10.1096/fj.201700247RR.
- Carter SL, Cibulskis K, Helman E, McKenna A, Shen H, Zack T, Laird PW, Onofrio RC, Winckler W, Weir BA. et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30(5):413–421. doi:10.1038/nbt.2203.
- Van Loo P, Nordgard SH, Lingjærde OC, Russnes HG, Rye IH, Sun W, Weigman VJ, Marynen P, Zetterberg A, Naume B. et al. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci USA. 2010;107(39):16910–16915. doi:10.1073/pnas.1009843107.
- Müller M, May S, Bird TG. Ploidy dynamics increase the risk of liver cancer initiation. Nat Commun. 2021;12(1):1896. doi:10.1038/s41467-021-21897-8.
- Zhou L, Jilderda LJ, Foijer F. Exploiting aneuploidy-imposed stresses and coping mechanisms to battle cancer. Open Biol. 2020;10(9):200148. doi:10.1098/rsob.200148.
- Guang MHZ, Kavanagh EL, Dunne LP, Dowling P, Zhang L, Bianchi G, Anderson KC, O’Gorman P, McCann A, Bianchi G. Targeting proteotoxic stress in cancer: a review of the role that protein quality control pathways play in oncogenesis. Cancers. 2019;11(1):66. doi:10.3390/cancers11010066.
- Iuliano L, Dalla E, Picco R, Mallavarapu S, Minisini M, Malavasi E, Brancolini C. Proteotoxic stress-induced apoptosis in cancer cells: Understanding the susceptibility and enhancing the potency. Cell Death Discovery. 2022;8(1):407. doi:10.1038/s41420-022-01202-2.
- Hosoya N, Miyagawa K. Targeting DNA damage response in cancer therapy. Cancer Sci. 2014;105(4):370–388. doi:10.1111/cas.12366.
- Hwang S, Gustafsson HT, O’Sullivan C, Bisceglia G, Huang X, Klose C, Schevchenko A, Dickson RC, Cavaliere P, Dephoure N. et al. Serine-dependent sphingolipid synthesis is a metabolic liability of aneuploid cells. Cell Rep. 2017;21(13):3807–3818. doi:10.1016/j.celrep.2017.11.103.
- Ben-David U, Amon A. Context is everything: Aneuploidy in cancer. Nat Rev Genet. 2020;21(1):44–62. doi:10.1038/s41576-019-0171-x.
- Lengauer C, Kinzler KW, Vogelstein B. Genetic instability in colorectal cancers. Nature. 1997;386(6625):623–627. doi:10.1038/386623a0.
- Alessi JV, Elkrief A, Ricciuti B, Wang X, Cortellini A, Vaz VR, Lamberti G, Frias RL, Venkatraman D, Fulgenzi CAM. et al. Clinicopathologic and genomic factors impacting efficacy of first-line chemoimmunotherapy in advanced NSCLC. J Thorac Oncol. 2023;18(6):731–743. doi:10.1016/j.jtho.2023.01.091.
- Bolhaqueiro ACF, Ponsioen B, Bakker B, Klaasen SJ, Kucukkose E, van Jaarsveld RH, Vivié J, Verlaan-Klink I, Hami N, Spierings DCJ. et al. Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat Genet. 2019;51(5):824–834. doi:10.1038/s41588-019-0399-6.
