Abstract
Human immunodeficiency virus (HIV)-infected individuals successfully treated for tuberculosis (TB) remain at risk of recurrence of the disease, especially in high TB incidence settings. We performed a systematic review, investigating whether secondary preventive therapy (sPT) with anti-TB drugs (preventive therapy in former TB patients with treatment success) is an effective strategy to prevent recurrence of TB in this patient group. We searched the databases PubMed, Cochrane Library, EMBASE, Web of Science and Google Scholar using the keywords HIV-infections, HIV, human immunodeficiency virus, AIDS, isoniazid, isoniazid preventive therapy (IPT), tuberculosis, TB, recurrence and recurrent disease, resulting in 253 potential publications. We identified eight publications for full text assessment, after which four articles qualified for inclusion in this systematic review. The quality of the included articles was rated using the GRADE system. All but one study were rated as having a high quality. In all included studies, sPT significantly decreased the incidence of recurrent TB in HIV-infected individuals to a substantial degree in comparison to non-treatment or placebo. Relative reductions varied from 55.0% to 82.1%. These data showed that the use of sPT to prevent recurrent TB in HIV-infected individuals was highly beneficial. These findings need to be confirmed in prospective studies with an adequate assessment of the effect of antiretroviral therapy (ART) and the occurrence of drug resistance.
Introduction
Tuberculosis (TB) and human immunodeficiency virus (HIV) epidemics are overlapping in many parts of the world, especially in sub-Saharan Africa.[Citation1] According to the World Health Organization (WHO), a minimum of one-third of the 37 million people living with HIV all over the world is infected with Mycobacterium tuberculosis (Mtb).[Citation1] An HIV infection is the strongest known risk factor for the development of a first episode of active TB. In HIV-infected individuals the risk of progressing to active TB following Mtb infection is 20–30 times higher compared to patients without HIV.[Citation1] An estimated 1.2 million new TB cases in HIV-infected individuals were notified globally in 2014, of which approximately 74% live in the African Region.[Citation2] TB is a leading cause of mortality among HIV-infected persons, accounting for 0.4 million HIV-associated TB deaths globally in 2014.[Citation2]
Active TB disease is proved to be preventable by offering primary preventive therapy. Primary preventive therapy aims to prevent a first episode of active TB in individuals in whom active TB has been excluded. In HIV-negative individuals the average preventive effect is a 60% reduction in the incidence of TB, while in HIV-infected individuals the average effect is around 30%.[Citation3,Citation4]
HIV-infected individuals who are not infected with a drug-resistant Mtb strain respond well to initial treatment. However, after successful treatment they remain at risk of recurrence of the disease, especially in settings with a high TB incidence. In 2014, pulmonary bacteriologically confirmed relapse/re-infection cases as a percentage of total case notifications were estimated at approximately 4.5% globally and 3.0% in the African region, although we know that previous treatment is markedly underestimated.[Citation2] Recurrence occurs through either re-infection with a new strain of Mtb or reactivation of the original Mtb strain (designated as relapse).
A potential approach to prevent recurrent TB is secondary preventive therapy (sPT) using anti-TB drugs. sPT aims to reduce the incidence of recurrent TB disease in former TB patients with treatment success. Until now, limited information on the efficacy of such an approach in HIV-infected individuals is available, with only a few studies conducted in the period 1995–2003. In these studies, the potential influence of plasma viral load, CD4 cell count and ART is often not addressed.
After 2003, the research focus gradually switched to the use of antiretroviral therapy (ART) as a preventive strategy for TB in HIV-infected individuals, neglecting sPT as a potential approach. The annual incidence of TB in HIV-infected individuals remains high despite the enormous increased use of ART.[Citation2,Citation5] It might be important to elucidate whether sPT in HIV-infected individuals is effective to prevent recurrence of TB after successful treatment of a first TB episode.
This systematic review investigates the existing evidence of the effect of sPT for the prevention of recurrent TB in HIV-infected individuals. In addition, the context will be explored in which this preventive treatment strategy could be used.
Methods
Search strategy
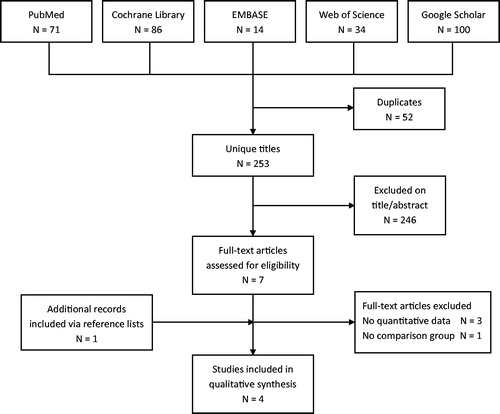
We searched the databases PubMed, Cochrane Library, EMBASE, Web of Science and Google Scholar based on an a priori defined review protocol and PICO. The PICO defines the search strategy in Patients, Intervention, Comparison and Outcome. The patient population included HIV-infected individuals, the intervention referred to sPT, the comparison included placebo/no preventive treatment, and the outcome was recurrent TB. The PICO and search strategy are summarised in . sPT was defined as preventive therapy in former TB patients with treatment success. Complete databases were explored without restrictions for year of publication. All types of study designs were included. There were no restrictions for age group, use of ART or level of immunosuppression.
Table 1. PICO and search strategies.
The search strategy contained the terms: HIV-infections, HIV, human immunodeficiency virus, AIDS, isoniazid, isoniazid preventive therapy (IPT), tuberculosis, TB, recurrence and recurrent disease.
Study selection
An assessment of titles and abstracts, and selection of full text articles were performed by two independent reviewers (WB and FvL). Inclusion criteria for full text selection were defined as (i) study conducted in humans, (ii) HIV-infected individuals (iii) secondary prevention, (iv) recurrent pulmonary TB, (v) comparison with placebo/no intervention and (vi) written in English language.
Reference lists of selected articles were explored manually to identify possible additional articles missed in the search strategy. Final assessment for inclusion was done in August 2015.
Data extraction and literature review
Data were extracted by a single author (WB) following a pre-defined data extraction protocol. Extracted data included main study characteristics, patient characteristics, disease characteristics and main outcomes (). The main outcome (incidence of recurrent TB) between study groups was compared in all four articles. The occurrence was measured as the rate or risk of recurrent TB, and the effect was measured as the relative rate or risk. Given the diverse interventions and study designs, we do not present a pooled estimate of the effect of sPT. Without such a pooled effect, it is not possible to anchor a funnel plot used to assess potential selection or publication bias.
Table 2. Extracted data from included studies.
Quality assessment
For quality assessment of included studies, the GRADE system was used. This system is developed by the Grades of Recommendation, Assessment, Development and Evaluation Working Group (GRADE Working Group) to ‘define the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the quantity of specific interest’.[Citation6]
The GRADE system rates quality of studies depending on the underlying methodology, directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias.
Results
The search strategy yielded eight full text articles and four included studies (). A summary of results of all individual, included studies is presented in .
Table 3. Summary of included studies.
Summary of individual studies
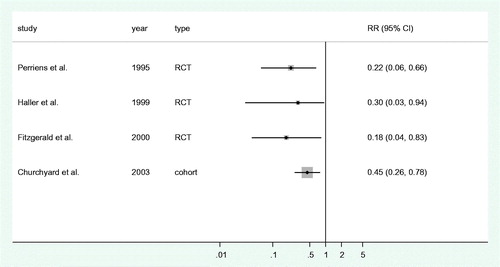
The study by Perriëns et al. [Citation7] describes a randomized, single-blind, placebo-controlled trial to assess the effect of extension of first-line TB treatment from six to 12 months among patients with an HIV infection. Patients received either isoniazid (INH) and rifampicin twice weekly for an additional six months after initial treatment (n = 121) or a placebo (n = 119). Patients receiving INH and rifampicin after initial treatment were significantly less likely to experience a recurrent episode of TB compared to those receiving a placebo (relative risk 0.21, p < .01).
Haller et al. describe an open-label, randomized trial assessing the effectiveness of INH and sulphadoxine–pyrimethamine in preventing recurrent TB in HIV-infected individuals having completed a full treatment course for pulmonary TB.[Citation8] The relative rate for the incidence of recurrent TB comparing patients receiving additional treatment (n = 134) and those not (n = 129) was 0.30 (95% confidence interval: 0.09–0.94).
Fitzgerald et al. [Citation9] describe an open-label, randomized trial comparing an additional year of INH after completion of initial treatment (n = 68) with an additional placebo (n = 74) in HIV-infected individuals. The intervention significantly reduced the incidence of recurrent TB from 7.8 per 100 person-years to 1.4 per 100 person-years (relative risk 0.18, 95% confidence interval: 0.04–0.83).
Churchyard et al. [Citation10] describe a cohort study assessing the effect of INH and cotrimoxazole (n = 338) versus cotrimoxazole only or no medication (n = 221) in HIV-infected gold miners in South Africa. The rate ratio for recurrent TB comparing the group with INH with the group without INH was 0.45 (95% confidence interval: 0.26–0.78).
Summary of outcome
In each of the four studies, sPT significantly decreased the incidence of recurrent TB in HIV-infected individuals (). None of the studies found a significant effect on overall survival, with the exception of Haller et al. [Citation8] who reported a mortality decline in the prophylaxis group in the first year of follow-up but not thereafter.
ART and CD4 cell counts
Information on the use of ART and CD4 cell counts is presented in . This information is limited or completely lacking in the included articles, probably due to the period and settings in which the studies were conducted.
Quality assessment
The quality of the four individual studies was assessed based on study design, methodology, directness, heterogeneity, precision and possible up- or downgrades. According to the GRADE methodology, studies were evaluated for a possible upgrade based on high effect sizes or dose-response gradients. The necessity of a potential downgrade was examined based on the risk of selection and ascertainment bias, and differential loss to follow-up. None of the reviewed studies qualified for either an up- or downgrade because of the reasons mentioned above.
There was a necessary downgrade of Churchyard et al. [Citation10] because the article does not state clearly from which population the patients in the intervention group were sampled. The article only states that the participants from the IPT cohort were derived from a cohort of HIV-infected men receiving INH indefinitely as part of a clinical trial, without referring to any specific study.
Final quality ratings were high for Perriëns et al., Haller et al. and Fitzgerald et al., and very low for Churchyard et al.
Discussion
In all four included articles, sPT decreased the incidence of recurrent TB in HIV-infected individuals to a substantial degree in comparison to non-treatment or placebo. Relative reductions varied from 55.0% to 82.1% (RR: 0.18–0.45). This suggests that the use of sPT to prevent recurrent TB in HIV-infected individuals may be highly beneficial. However, this conclusion needs careful consideration.
Duration and drug choice
For the appraisal of such an approach currently we have to rely on issues discussed in primary preventive therapy due to the lack of evidence on outstanding issues in sPT. As with primary preventive therapy, the ideal duration of, and the most effective drugs used for sPT are yet unclear. In the four studies included in this systematic review, the duration of sPT varied from six up to 24 months, while treatment regimens contained INH with or without rifampicin.[Citation7,Citation8] The studies represent different strategies with separate mechanisms for sPT: either treatment continuation in order to further reduce the reservoir of remaining Mtb, or provision of a specific preventive treatment regimen against re-infection among persons with a high risk of developing active disease. In our review, both strategies have a similar effect on the main outcome measure (recurrent TB). The reported data do not allow for a thorough assessment of these strategies with respect to adverse events or treatment adherence. Differences in reported frequencies in adverse events could be solely due to differences in study procedures, differential ascertainment or reporting bias.
The duration of effectiveness of sPT remains unclear as well because of short follow-up periods in the included studies. Optimal treatment duration has been explored in primary preventive therapy in latently infected HIV-infected individuals. WHO guidelines recommend an IPT regimen for at least six months in HIV-infected adults and adolescents with positive or unknown tuberculin skin test (TST) status.[Citation11] More recent evidence suggests an extension of treatment in settings with a high infection pressure of TB. In Botswana, a randomized, double-blind, placebo-controlled trial was conducted between 2004 and 2009 that compared 36-months continuous IPT to a six-months regimen, showing a reduced incidence of TB of 43% in the continuous IPT group (74% among TST positive individuals).[Citation12] Nevertheless, TB incidence increased by 90% after cessation of the 36-month treatment, while in the six-month group TB incidence reached baseline level after approximately 200 days.[Citation13] The rate of this increase in risk is likely to depend on the infection pressure of TB in the population.
For primary preventive therapy, the treatment regimen used did not seem to have a large effect on the effectiveness of the strategy.[Citation4] However, using mathematical modelling in primary preventive therapy settings, Houben et al. showed that INH alone does not cure latent Mtb infections in most individuals.[Citation14] The article states that continuous IPT is advised in high-burden settings and should be integrated in HIV care, where in low-incidence settings a different regimen with multiple drugs, e.g. INH plus rifampicin, is recommended to reach complete cure. Continuous IPT in all patients may not be feasible, making it worthwhile to assess if sPT for a limited duration with INH (with or without additional drugs) can be considered as an initial intervention to reduce incident TB in patients with treatment success for active TB.
Selection of drug resistance
The risk of selection of drug-resistant strains of Mtb by using IPT has been a point of concern and one of the reasons for reluctance on large-scale implementation of IPT in HIV-infected individuals. A meta-analysis including published data from 1951 till 2005 did not find a statistically significant association between IPT use and drug resistance.[Citation15] Two articles with more recent data, one of them using mathematical modelling, confirm the minimal effect of IPT on selection of INH-resistant Mtb stains.[Citation16,Citation17] The studies included in our review do not report any substantial selection. However, the last included study was published in 2003. It is unclear whether this finding holds in the present-day situation with more TB drug resistance.
In contrast, a study using whole genome sequencing of TB isolated from macaques described that Mtb mutations continue in the same frequency during TB latency as during active disease despite large differences in bacterial replications.[Citation18] This suggests that patients with latent disease theoretically might select drug-resistant Mtb strains when using prophylactic mono-therapy with INH. In addition, a mathematical modelling study reported the potential of amplifying resistance at a population level during community-wide use of IPT due to selective suppression of INH-susceptible Mtb, advancing INH-resistant strains. This despite an absence of effect of IPT on INH resistance at an individual level.[Citation19] The same study showed that this potential effect of community-wide use of IPT in increased drug resistance at population level occurred over a period of decades, indicating that this should not discourage the use of IPT, especially when combined with adequate diagnosis and timely treatment.[Citation20]
sPT and ART
Recommendations on sPT must be seen in the context of the increasing use of ART, which has shown to decrease the incidence of TB in HIV-infected individuals markedly.[Citation21–23] Some reports suggest an additional effect of primary preventive therapy in addition to ART. A cohort study from Rio de Janeiro (Brazil) including 8129 HIV-infected individuals starting ART, reported that the combination of ART and IPT was associated with a lower TB incidence compared to ART only.[Citation24] A similar finding was reported from a randomized, double blinded, placebo-controlled trial in South Africa, and in a large randomized trial assessing early initiation of ART.[Citation25,Citation26]
The potential additional effect of ART on primary preventive therapy of TB lies in the improved immunological status of the HIV-infected individuals. A similar effect of ART can be envisioned in the context of sPT. It is the deterioration of the immune status caused by HIV-infection that increases the risk for progression to active TB disease. It has been speculated that HIV-infection might also increase the risk of TB-infection itself.
In three of the four included studies in this review, it remains unclear whether HIV-infected study participants received ART during their follow-up period. Most likely, none or just a limited number of the participants received ART because the studies were conducted in a period in which access to ART was generally limited. The potential role of sPT thus needs to be corroborated in settings where HIV-infected individuals are provided with ART, including on the risk of drug–drug interactions and adverse events such as neurotoxicity.[Citation27,Citation28]
CD4 cell counts
The overall potential effect of CD4 cell counts on the incidence of recurrent TB could not be assessed in our study. This was due to the absence of detailed information in most of the studies, as well as the different timing within the treatment at which CD4 cell counts were available (either at the start of initial anti-tuberculosis therapy or at the start of sPT).
Relapse versus re-infection
None of the included studies made a distinction between relapse or re-infection as the mechanism of recurrent TB. DNA-fingerprinting studies showed that relapse is more likely to occur shortly after therapy cessation, and re-infection in the long run.[Citation29,Citation30] Furthermore, the contribution of relapse and re-infection to recurrent TB differs by geographical area and will be driven by differences in prevalence of the disease in the general population. However, the frequency of re-infection in patients with recurrent disease after treatment success can be substantial (15% Uganda, 27% Malawi, 30% Brazil, 50–77% South Africa).[Citation31–34]
Limitations
A limitation of this review is the possibility of recurrent TB diagnosis being only based on clinical symptoms in Haller et al. and Fitzgerald et al., which could increase the risk of overestimation of recurrent TB in these studies. However, we do not think that this method of evaluating recurrent TB will undermine the overall conclusion of the positive effect on the main outcome found in our review. Another limitation is the low number of articles eligible to be included, hampering strong conclusions. However, the quality of systematic reviews does not depend on the number of articles included, as exemplified by the Cochrane group publishing results for reviews without any eligible article. When the scientific question underlying the review is sound, and the followed methodology according to set standards, the results of a systematic review can inform the scientific community.
Recommendations
Although sPT showed to have a beneficial effect in the prevention of recurrent TB in HIV-infected individuals successfully completing their TB-treatment, the implementation of sPT in communities might be a complex procedure, as it is with primary preventive therapy.[Citation35] There will be several approaches possible. sPT could be given continuously to HIV-infected individuals as Houben et al. suggests in initial prevention settings.[Citation14] sPT could also be given as an extension of treatment for initial, active TB.
This review can inform national TB programs and health authorities on their prophylactic treatment strategies for HIV-infected individuals. To confirm the beneficial effect of sPT in HIV-infected individuals prospective clinical trials are needed with an adequate assessment of the effect of ART and the occurrence of drug resistance.
Disclosure statement
The authors declare no conflict of interest.
Funding
No funding was received to perform the review or to write the manuscript.
References
- WHO. Tuberculosis fact sheet no. 104. [revised 2016 Oct 16]. 2016. Available from: http://www.who.int/mediacentre/factsheets/fs104/en.
- WHO. Global tuberculosis report 2015. [revised 2016 Oct 8]. Available from: http://who.int/tb/publications/global_report/en.
- Smieja MJ, Marchetti CA, Cook DJ, Smaill FM. Isoniazid for preventing tuberculosis in non-HIV infected persons. Cochrane Database Syst Rev. 2000;CD001363. doi: 10.1002/14651858.CD001363.
- Akolo C, Adetifa I, Shepperd S, Volmink J. Treatment of latent tuberculosis infection in HIV infected persons. Cochrane Database Syst Rev. 2010;CD000171. doi: 10.1002/14651858.CD000171.pub3.
- AIDS by the numbers, Geneva, 2016. Available from: http://www.unaids.org/sites/default/files/media_asset/AIDS-by-the-numbers-2016_en.pdf.
- Higgins JPT, Green S (Eds). Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: http://www.handbook.cochrane.org.
- Perriëns JH, St Louis ME, Mukadi YB, et al. Pulmonary tuberculosis in HIV-infected patients in Zaire. A controlled trial of treatment for either 6 or 12 months. N Engl J Med. 1995;332:779–784.
- Haller L, Sossouhounto R, Coulibaly IM, et al. Isoniazid plus sulphadoxine-pyrimethamine can reduce morbidity of HIV-positive patients treated for tuberculosis in Africa: a controlled clinical trial. Chemotherapy. 1999;45:452–465.
- Fitzgerald DW, Desvarieux M, Severe P, et al. Effect of post-treatment isoniazid on prevention of recurrent tuberculosis in HIV-1-infected individuals: a randomised trial. Lancet. 2000;356:1470–1474.
- Churchyard GJ, Fielding K, Charalambous S, et al. Efficacy of secondary isoniazid preventive therapy among HIV-infected Southern Africans: time to change policy?. AIDS. 2003;17:2063–2070.
- WHO. Intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. [revised 2016 Oct 16]. 2011. Available from: http://www.who.int/hiv/pub/tb/9789241500708/en.
- Samandari T, Agizew TB, Nyirenda S, et al. 6-month versus 36-month isoniazid preventive treatment for tuberculosis in adults with HIV infection in Botswana: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:1588–1598.
- Samandari T, Agizew TB, Nyirenda S, et al. Tuberculosis incidence after 36 months’ isoniazid prophylaxis in HIV-infected adults in Botswana: a posttrial observational analysis. AIDS. 2015;29:351–359.
- Houben RMGJ, Sumner T, Grant AD, et al. Ability of preventive therapy to cure latent Mycobacterium tuberculosis infection in HIV-infected individuals in high-burden settings. Proc Natl Acad Sci USA. 2014;111:5325–5330.
- Balcells ME, Thomas SL, Godfrey-Faussett P, et al. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerging Infect Dis. 2006;12:744–751.
- Van Halsema CL, Fielding KL, Chihota VN, et al. Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. AIDS. 2010;24:1051–1055.
- Basu S, Maru D, Poolman E, et al. Primary and secondary tuberculosis preventive treatment in HIV clinics: simulating alternative strategies. Int J Tuberc Lung Dis. 2009;13:652–658.
- Ford CB, Lin PL, Chase MR, et al. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet. 2011;43:482–486.
- Mills HL, Cohen T, Colijn C. Community-wide isoniazid preventive therapy drives drug-resistant tuberculosis: a model-based analysis. Sci Transl Med. 2013;5:180ra49.
- Mills HL, Cohen T, Colijn C. Response to comment on “Community-wide isoniazid preventive therapy drives drug-resistant tuberculosis: a model-based analysis”. Sci Transl Med. 2013;5:204lr4.
- Miranda A, Morgan M, Jamal L, et al. Impact of antiretroviral therapy on the incidence of tuberculosis: the Brazilian experience, 1995–2001. PLoS One. 2007;2:e826.
- Girardi E, Antonucci G, Vanacore P, et al. Impact of combination antiretroviral therapy on the risk of tuberculosis among persons with HIV infection. AIDS. 2000;14:1985–1991.
- Santoro-Lopes G, de Pinho AMF, Harrison LH, et al. Reduced risk of tuberculosis among Brazilian patients with advanced human immunodeficiency virus infection treated with highly active antiretroviral therapy. Clin Infect Dis. 2002;34:543–546.
- Golub JE, Saraceni V, Cavalcante SC, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21:1441–1448.
- Rangaka MX, Wilkinson RJ, Boulle A, et al. Isoniazid plus antiretroviral therapy to prevent tuberculosis: a randomised double-blind, placebo-controlled trial. Lancet. 2014;384:682–690.
- TEMPRANO ANRS 12136 Study Group, Danel C, Moh R, Gabillard D, et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373:808–822.
- Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18:388–399.
- Forget EJ, Menzies D. Adverse reactions to first-line antituberculosis drugs. Expert Opin Drug Saf. 2006;5:231–249.
- Sonnenberg P, Murray J, Glynn JR, et al. HIV-1 and recurrence, relapse, and reinfection of tuberculosis after cure: a cohort study in South African mineworkers. Lancet. 2001;358:1687–1693.
- Marx FM, Dunbar R, Enarson DA, et al. The temporal dynamics of relapse and reinfection tuberculosis after successful treatment: a retrospective cohort study. Clin Infect Dis. 2014;58:1676–1683.
- Luzze H, Johnson DF, Dickman K, et al. Relapse more common than reinfection in recurrent tuberculosis 1-2 years post treatment in urban Uganda. Int J Tuberc Lung Dis. 2013;17:361–367.
- Guerra-Assunção JA, Houben RMGJ, Crampin AC, et al. Recurrence due to relapse or reinfection with Mycobacterium tuberculosis: a whole-genome sequencing approach in a large, population-based cohort with a high HIV infection prevalence and active follow-up. J Infect Dis. 2015;211:1154–1163.
- Unis G, Ribeiro AW, Esteves LS, et al. Tuberculosis recurrence in a high incidence setting for HIV and tuberculosis in Brazil. BMC Infect Dis. 2014;14:548
- Verver S, Warren RM, Beyers N, et al. Rate of reinfection tuberculosis after successful treatment is higher than rate of new tuberculosis. Am J Respir Crit Care Med. 2005;171:1430–1435.
- Getahun H, Granich R, Sculier D, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS. 2010;24:S57–S65.