Abstract
Background: The aim of this study was to evaluate the positive predictive value (PPV) of ELISpot in bronchoalveolar lavage (BAL) and pleural fluid for the diagnosis of active tuberculosis (TB) in real-life clinical practice, together with the added value of a cut-off >1.0 for the ratio between the extra-sanguineous and systemic interferon-gamma responses in positive samples.
Methods: A retrospective, single-centre study was performed. Patients with positive ELISpot in BAL and pleural fluid were included.
Results: The PPV for TB in patients with positive ELISpot in BAL (n = 40) was 64.9%, which increased to 82.6% for the ESAT-6 panel and 71.4% for the CFP-10 panel after the introduction of a cut-off >1.0 for the ratio between the BAL and blood interferon-gamma responses. In patients with positive ELISpot in pleural fluid (n = 16), the PPV for TB was 85.7%, which increased to 91.7% for the ESAT-6 panel and 92.3% for the CFP-10 panel after the introduction of a cut-off >1.0 for the ratio between the pleural fluid and blood interferon-gamma responses.
Conclusions: This report describes the PPV of ELISpot in BAL and pleural fluid for the diagnosis of active TB in real-life clinical practice. The results indicate the possibility of an increase of the PPV using a cut-off >1.0 for the ratio between the extra-sanguineous and systemic interferon-gamma responses. Further studies are needed to underline this ratio-approach and to evaluate the full diagnostic accuracy of ELISpot in extra-sanguineous fluids like BAL and pleural fluid.
Introduction
Tuberculosis (TB) results in high morbidity and mortality worldwide, and rapid diagnosis is still a major challenge. In the Netherlands, 25% of patients are treated for TB without a definitive diagnosis due to negative acid-fast staining, polymerase chain reaction (PCR) and culture, indicating the need for additional diagnostic tools.
In recent years, Mycobacterium tuberculosis (MTB)-specific interferon-gamma release assays (IGRA) in extra-sanguineous fluids have been investigated for use in the diagnosis of TB, based on the theory that MTB-specific T-lymphocytes are concentrated at the site of infection due to homing and antigen-specific proliferation.[Citation1] A systematic review summarized the current evidence about the diagnostic accuracy of these tests.[Citation2] Concerning T-SPOT.TB (Enzyme-Linked ImmunoSpot assay, ELISpot) in extra-sanguineous fluids, the pooled sensitivity and specificity were 88% and 82%, respectively. For Quantiferon Gold-in tube in extra-sanguineous fluids, this was 48% and 82%, respectively. Another systematic review about the diagnostic performance of MTB-specific IGRA in pleural fluid combined the results of ELISpot and Quantiferon Gold-in tube, resulting in the pooled sensitivity and specificity of 72% and 78%, respectively.[Citation3,Citation4] The results of both reviews indicate the diagnostic superiority of ELISpot compared with Quantiferon Gold-in tube in extra-sanguineous fluids; however, the clinical utility of IGRA in extra-sanguineous fluids is still under research. Especially, the limited positive predictive value (PPV), although not evaluated in both reviews, seems a limitation of this test as in a large, multicentre study in a low TB-endemic area, the PPV of MTB-specific IGRA in bronchoalveolar lavage (BAL) was only 55%.[Citation5]
In active TB, frequencies of interferon-gamma producing MTB-specific T-lymphocytes concentrated at the site of infection are shown to be higher compared with frequencies of interferon-gamma producing MTB-specific T-lymphocytes in peripheral blood,[Citation1,Citation5,Citation6] probably resulting in a ratio of >1.0 between the extra-sanguineous and systemic interferon-gamma responses. The introduction of a cut-off ratio >1.0 between the extra-sanguineous and systemic interferon-gamma responses could theoretically increase the PPV of IGRA in extra-sanguineous fluids. This item is not addressed in either review [Citation2,Citation3]; however, a cut-off has been suggested by some reports.[Citation1,Citation7–9] These studies included limited number of subjects and, moreover, three of these studies [Citation7–9] were from a high TB-endemic area, making it difficult to extrapolate the results to lower endemic areas.
In our hospital, the application of MTB-specific IGRA (ELISpot) in extra-sanguineous fluids is part of the diagnostic evaluation in patients with a clinical suspicion of active TB. The aim of this study was to evaluate the PPV of ELISpot in BAL and pleural fluid for the diagnosis of active TB in real-life clinical practice, together with the added value of a cut-off >1.0 for the ratio between the extra-sanguineous and systemic interferon-gamma responses in positive samples.
Material and methods
Patients
A retrospective, single-centre study was performed in a hospital in Utrecht, The Netherlands. All patients with positive ELISpot in BAL and pleural fluid from 2006 to 2012 of whom concurrent ELISpot blood results were available (in order to allow the ratio-analysis between the extra-sanguineous and systemic interferon-gamma responses) were included, with a follow-up of at least two years. Patients with negative or indeterminate ELISpot in BAL and pleural fluid were excluded. Patient characteristics, including age, gender, comorbidities, medication, smoking habits, country of origin, travel history, BCG vaccination status, tuberculin skin test (TST) results, Human immunodeficiency virus (HIV) status, clinical signs and symptoms, laboratory results, thoracic imaging results, microbiological results and blood ELISpot-assay results were examined from medical records.
Diagnostic work-up
In TB suspect patients, the regular work-up consisted of blood examination for ELISpot TB and inflammatory markers (including leucocyte count and C-reactive protein levels) together with thoracic imaging (X-ray or computed tomography (CT)-scan). In all patients suspected of having pulmonary TB but who were unable to cough up sputum or with three samples smear-negative sputum (not special morning sputum), BAL was performed with 150 mL saline fluid placed into an affected lung segment. Gastric lavages were not used. In patients suspected of tuberculous pleuritis, 20 mL pleural fluid was collected, of which 10 mL was used for PCR and culture. Diagnostic tests performed at BAL and pleural fluid included always MTB-specific IGRA (ELISpot), acid-fast staining using auramine, PCR for MTB, standard culture for bacterial pathogens and mycobacterial culture. For PCR technique, prior to DNA extraction, samples underwent a pre-extraction preparation method using a combination of N-acetyl-l-cysteine and sodium hydroxide (NALC–NaOH).[Citation10] DNA was isolated using Magnapure LC (Roche, Almere, the Netherlands) according to the manufacturer’s instructions. The MTB-specific PCR was performed using primer set 2 targeted on the IS6110 sequence. PCR was performed using the ABI Prism 75,000 sequence detection system (Applied Biosystems, Foster City, CA).[Citation11] For mycobacterial culture, specimens were inoculated using a MGIT system (Becton Dickinson, Shannon, Ireland). Positive cultures were verified using acid-fast staining and PCR for MTB. Positive cultures were subsequently referred to the National Institute for Public Health and the Environment for identification and antimicrobial susceptibility testing.
Interferon-gamma release assay
MTB-specific IGRA (ELISpot) in peripheral blood mononuclear cells (PBMCs) was performed using the T-SPOT.TB platform, according to the manufacturer’s instructions (Oxford Immunotec Ltd., Abingdon, UK). Briefly, 2.5 × 105 fresh PBMCs were incubated with 50 μL of AIM-V medium (negative control), phytohemagglutinin (PHA, positive control) and two MTB-specific antigens (ESAT-6/panel A, and CFP-10/panel B, respectively). After 16–20 h of incubation at 37 °C and 5% CO2, the microtitre plates were washed and a conjugate incubation followed by a detection step was carried out to visualize the interferon-gamma production by sensitized T-cells. Spot forming cells (SFCs) were enumerated using the ELISpot reader (Auto Immun Diagnostika GmbH, Strassberg, Germany). Definition of the results (positive, negative or indeterminate) was according to the manufacturer’s instructions and as described previously.[Citation5,Citation12] Borderline zones were not used.
ELISpot in BAL and pleural fluid was performed as described previously.[Citation5] Time from sampling to start analysis was within three hours. Fresh cells were isolated from BAL and pleural fluid, and if possible, 2.5 × 105 fresh cells were incubated and processed according to the protocol described above. In the absence of validated cut-off values for ELISpot in BAL and pleural fluid, positive, negative and indeterminate results were defined in agreement with ELISpot in blood.
Tuberculosis case definition
According to the WHO guidelines,[Citation13] the definition of TB cases resulted in four possible diagnoses: definite, probable, uncertain or no active TB. Definite TB was diagnosed in patients with clinical findings compatible with TB, confirmed with positive culture and/or PCR. Patients with signs and symptoms of TB without laboratory confirmation (negative PCR and culture) but who recovered after treatment with tuberculostatic drugs were defined as probable TB. Patients with definite TB as well as patients with probable TB are regarded to be patients with active TB in real-life clinical practice. Patients lost to follow-up and patients with an unclear clinical course in whom no laboratory confirmation of active TB could be obtained were classified as uncertain TB. Finally, in cases with another diagnosis, the case was defined as no active TB, including patients with only latent TB infection (LTBI), defined as patients with positive ELISpot in blood, but without arguments for active disease. Development of active TB was assessed with respect to at least two years of follow-up. TB case definition was made with the consensus of three of the authors (A.W.J.B., A.S.R.v.L. and R.W.H.), in collaboration with an independent TB expert who was not aware of the inclusion criteria of this study (W.C.M.d.L.). This TB expert evaluated all cases retrospectively, with access to all anonymized data except results of ELISpot in BAL and pleural fluid, ensuring an independent TB case definition.
Analysis
Baseline clinical characteristics were described as medians and ranges in non-normally distributed variables and percentages for categorical variables.
The PPV of ELISpot without ratio was calculated for positive BAL and pleural fluid samples separately, dividing the number of patients with active (definite and probable) TB by the total number of patients with positive ELISpot in BAL respectively pleural fluid.
The ratio between the extra-sanguineous and systemic interferon-gamma responses (hereinafter referred to as ‘ratio’) was calculated dividing the number of SFCs in BAL respectively pleural fluid by the number of SFCs in blood, for ESAT-6 and CFP-10 panels separately, because of the differences in SFCs between ESAT-6 and CFP-10 panels.
The PPV using a cut-off >1.0 for the ratio was calculated for the ESAT-6 panel and the CFP-10 panel separately, dividing the number of patients with active TB and ratio >1.0 by the total number of patients with positive ELISpot in BAL respectively pleural fluid and ratio >1.0.
Furthermore, the PPV using a cut-off >1.0 was calculated in case of ratio >1.0 in at least one of the two panels (either ESAT-6 or CFP-10), dividing the number of patients with active TB and ratio >1.0 in at least one of the two panels by the total number of patients with positive ELISpot in BAL respectively pleural fluid and ratio >1.0 in at least one of the two panels.
Finally, the PPV using a cut-off >1.0 was calculated in case of ratio >1.0 in the ESAT-6 and CFP-10 panel simultaneously, dividing the number of patients with active TB and ratio >1.0 in both panels simultaneously by the total number of patients with positive ELISpot in BAL respectively pleural fluid and ratio >1.0 in both panels simultaneously.
In evaluation of the PPV (with and without ratio), patients with uncertain TB were excluded, as most of them were lost to follow-up and definite case definition was not possible.
Because of the limited sample size, evaluation of the statistical significance of the added value of a cut-off ratio >1.0 at PPV is not suitable in this study. This study is aimed as a hypothesis generating report and should be followed by a well-powered prospective study.
Data were analysed using SPSS statistics version 21.0 (Armonk, NY). Figures were composed with the aid of GraphPad Prism 6.0 (La Jolla, CA).
Results
Patients, case definition and diagnostic results
All eighty-one patients with positive ELISpot in BAL and pleural fluid from 2006 to 2012 were evaluated. Twenty-five patients with positive ELISpot in BAL or pleural fluid could not be enrolled, because of the absence of concurrent blood ELISpot blood results. Fifty-six patients with positive ELISpot in BAL or pleural fluid were included, of which three were lost to follow-up. Thirty-eight patients were male. Median age was 48 years. Of all patients, 35 were born in a TB-endemic area (defined as countries with an annual TB incidence of >50/100,000),[Citation13] and most of them are likely to be BCG-vaccinated, although the BCG-vaccination details were not recorded in most of the medical files. Five patients had TB in their history. None of the patients were HIV-positive and only two patients were using immunosuppressive medication (one patient was treated with 5 mg prednisone daily; another patient was treated with a combination of 15 mg prednisone daily and methotrexate). None of the patients not receiving treatment developed TB during follow-up. The patients’ baseline characteristics are summarized in .
Table 1. Patients’ baseline characteristics.
Forty patients had positive ELISpot in BAL. Sixteen patients (separate cases) had positive ELISpot in pleural fluid as shown in . After assessment of the cases together with the independent TB expert, twenty-three patients (41.1%) were classified with definite TB, (including six patients with definite tuberculous pleuritis). Thirteen patients (23.2%) were classified with probable TB (including six patients with probable tuberculous pleuritis). Five patients (8.9%) were classified with uncertain TB, of whom three were lost to follow-up. Another fifteen patients (26.8%) were classified with no signs of active TB, although most of them (n = 11) had LTBI because of positive ELISpot in blood, and .
Table 2. Results of ELISpot in BAL and pleural fluid with respect to the tuberculosis case definition.
Table 3. Results of ELISpot in blood; results of auramine, PCR and culture for MTB in BAL and pleural fluid.
Other diagnostic results (including auramine, PCR and culture for MTB and ELISpot in blood) are summarized in , with respect to the TB case definition. In only one patient, resistance to isoniazid was established; all other MTB strains showed sensitivity to all tuberculostatic drugs tested.
Positive predictive value of ELISpot in BAL and pleural fluid and the added value of a cut-off >1.0 for the ratio between the extra-sanguineous and systemic interferon-gamma responses
The PPV of ELISpot in BAL fluid for active TB was 64.9% (=24/37), . The PPV of ELISpot in pleural fluid for active TB was 85.7% (=12/14), .
The calculated ratios between the extra-sanguineous and systemic interferon-gamma responses varied from 0.1 to almost 300 and are represented in (ESAT 6-panel) and (CFP-10 panel), for BAL and pleural fluid in separate colors. In patients with no active TB, patients with and without LTBI were represented separately.
Figure 1. Ratio between SFCs in BAL (black dots) respectively pleural fluid (red dots) and SFCs in blood (y-axis, scale log 10), with respect to the tuberculosis (TB) case definition (x-axis). Results of ESAT-6 (a) and CFP-10 (b) are shown separately. In patients with no active TB, patients with LTBI (filled dots) and without LTBI (open dots) were represented separately.
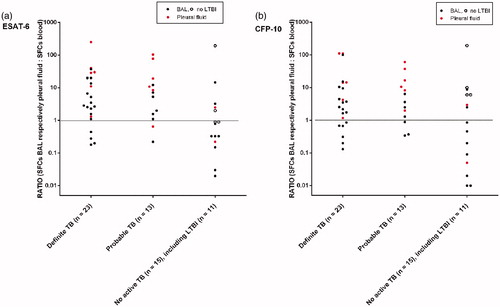
For positive ELISpot in BAL, a ratio of >1.0 resulted in a PPV for active TB of 82.6% (=19/23) for the ESAT-6 panel () and 71.4% (=15/21) for the CFP-10 panel (). A ratio of >1.0 in at least one of the two panels (either ESAT-6 or CFP-10) resulted in a PPV for active TB of 73.1% (=19/26). A ratio of >1.0 in the ESAT-6 and CFP-10 panel simultaneously resulted in a PPV of 83.3% (=15/18), .
Figure 2. PPV of ELISpot in BAL fluid (a) and pleural fluid (b) (first column) together with the added value of cut-off >1.0 for the ratio between the extra-sanguineous and systemic interferon-gamma responses in the CFP-10 panel (second column), in the ESAT-6 panel (third column), in at least one of the two panels (fourth column) and in the ESAT-6 and CFP-10 panel simultaneously (fifth column).
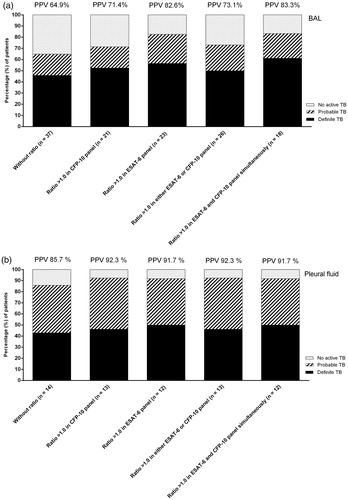
For positive ELISpot in pleural fluid, a ratio of >1.0 resulted in a PPV for active TB of 91.7% (=11/12) for the ESAT-6 panel () and 92.3% (=12/13) for the CFP-10 panel (). A ratio of >1.0 in at least one of the two panels (either ESAT-6 or CFP-10) resulted in a PPV for active TB of 92.3% (=12/13). A ratio of >1.0 in the ESAT-6 and CFP-10 panel simultaneously resulted in a PPV of 91.7% (=11/12), .
Discussion
This retrospective study evaluates the PPV of ELISpot in BAL and pleural fluid for the diagnosis of active TB in real-life clinical practice. The results indicate the possibility of an increase of the PPV using a cut-off >1.0 for the ratio between the extra-sanguineous and systemic interferon-gamma responses. Because of the limited sample size, the data are not appropriate for statistical tests. As a result, recommendations about the most appropriate panel (ESAT-6, CFP-10 or a combination of both panels) for ratio-approach cannot be determined thus far. Nevertheless, the results are noteworthy and contain important lessons for future studies on the value of ELISpot in extra-sanguineous fluids.
In theory, a positive ELISpot in BAL or pleural fluid together with a ratio >1.0 between extra-sanguineous and systemic interferon-gamma responses could be helpful in the decision to initiate anti-TB treatment in patients with negative PCR MTB results and pending or negative culture results, but this hypothesis needs further, carefully designed, prospective studies to determine the definite diagnostic accuracy and the impact on treatment decision. In this study, it was not possible to evaluate the impact of ELISpot results at treatment decision.
Furthermore, this approach could prove to be valuable in the diagnosis of extrapulmonary difficult-to-diagnose TB (e.g. TB-meningitis),[Citation14] although this is difficult to study in lower endemic areas because of the low prevalence of TB-meningitis.
This approach is not intended to replace PCR and/or culture diagnostics, which remain essential in identifying the TB-strain and in susceptibility testing. Independently of the positioning of the value of ELISpot in extra-sanguineous fluids, it remains very important to improve PCR-technique (which is an ongoing process in recent years) and to strive for better (sputum) samples, including induced sputum, all aimed to improve rapid TB-diagnosis.
An important requirement for ELISpot in BAL and pleural fluid is the access to bronchoscopy and ELISpot procedure, which might be a limitation in lower income countries, but usually is not a problem in more developed countries.
As far as we know, this study is the first to explicitly address the PPV of ELISpot in BAL and pleural fluid together with the added value of a cut-off >1.0 for the ratio between the extra-sanguineous and systemic interferon-gamma responses in positive samples. Another strength of this study is the TB case definition according to the WHO guidelines together with the input of an external, blinded expert.
This study has several limitations, partially due to the retrospective and single-centre character of the study, which influences the possibilities to generalize the results.
Another limitation refers to the selection strategy. In clinical practice, in patients with pulmonary TB diagnosed with positive sputum samples, usually bronchoscopy is not performed and as a consequence ELISpot BAL cannot be obtained. As a result, these patients were not included in the study. Furthermore, as a result of the inclusion of only positive ELISpot in BAL and pleural fluid, full diagnostic accuracy of the test including the negative predictive value, sensitivity and specificity could not be evaluated. As preceding prospective studies addressed this issue already,[Citation2,Citation3] and we especially aimed to investigate the added value of the ratio-approach for the PPV, evaluation of full diagnostic accuracy was not the intention of this retrospective study.
Results may be influenced by the disease prevalence in the included population. Although the study was performed in a low incidence country, several patients were at a higher risk of TB because of ethnicity or as a result of travelling to endemic areas.
Due to the low incidence of tuberculosis, this study included relatively few patients during a longer period of time where changes in staff and diagnostic facilities might have affected the results. Another reason for the limited sample size is the exclusion of patients without concurrent ELISpot blood results.
The exclusion of patients with uncertain TB in the evaluation of the PPV may have affected the results as well, although the influence of only five patients probably is not substantial. Because of the absence of a definite diagnosis in these five patients, the effect of the exclusions at the results is unclear.
The lower PPV of ELISpot in BAL compared with PPV of ELISpot in pleural fluid could be explained by the differences in the type of fluid. BAL fluid is the result of lavage of a lung segment, resulting in dilution of the sputum. Furthermore, the collection of BAL fluid results in a higher risk of blood mixture because of possible irritation of the endobronchial mucosa during the procedure.
Consequently, the ratio-approach is expected to increase the PPV of ELISpot especially in BAL, as is indicated in our results. As a consequence of the inherent differences in yield during the collection of BAL fluid, it is difficult to standardize the ELISpot procedure in BAL fluids. In three patients, less than 2.5 × 105 cells could be harvested and incubated for ELISpot procedure. None of these patients did have active TB. Although this is a limitation of the study, it is unlikely that the results of these three patients have significantly influenced the results.
The PPV of ELISpot in BAL fluid in this study (64.9%) is somewhat higher compared with PPV of ELISpot in BAL fluid (55%) in previous mentioned prospective multi-centre study,[Citation5] possibly influenced by differences in TB-endemic ethnicity of the included patients, although this information is lacking in the mentioned paper.[Citation5] An influence of the differences in selection strategy between both studies cannot be excluded. Both studies emphasizes that PPV without ratio between extra-sanguineous and systemic interferon-gamma responses is a limitation of ELISpot in BAL.
Although promising, this ‘ratio-approach’ did not distinguish active from latent TB in all patients, which remains a limitation of the test. Five patients with positive ELISpot in BAL fluid and ratio ≤1.0 in ESAT-6 and CFP-10 panels simultaneously did have active TB. Three of these patients had mainly extrapulmonary TB with only minor pulmonary abnormalities, necessitating caution when interpreting BAL results in patients with mainly extrapulmonary TB. The two other patients with a ratio ≤1.0 in ESAT-6 and CFP-10 panels simultaneously definitely had pulmonary TB, one with a positive culture for M. bovis and the other with a positive culture for MTB. In contrast, five patients with positive ELISpot in BAL or pleural fluid and ratios of >1.0 in ESAT-6 and CFP-10 panels simultaneously did not have active TB. Three of these patients had negative ELISpot in their blood and just slightly positive ELISpot in BAL fluid. One patient also had a negative ELISpot in her blood, but with a convincingly positive ELISpot in BAL fluid and 191 spots in ESAT-6 and CFP-10 panels simultaneously, without arguments for active TB. She frequently travelled to a TB-endemic area, so the influence of possible TB exposure and subsequent immune activation in this ‘false-positive’ ELISpot in BAL and consequently ‘false-negative’ ELISpot in blood could be considered. One patient had ratio of >1.0 in the ESAT-6 and CFP-10 panels simultaneously, with positive ELISpot in blood and pleural fluid, although without evidence of active TB.
Evaluating the PPV of ELISpot in BAL and pleural fluid, it is instructive to be aware of patients with negative ELISpot in BAL or pleural fluid yet with active TB. Therefore, we evaluated all patients treated for TB in our hospital from 2006 until 2012. This resulted in the identification of one patient with definite TB in mediastinal lymph nodes (negative Auramine and PCR for MTB, but positive culture), without abnormalities in lung parenchyma and consequently negative ELISpot BAL.
In conclusion, this report describes the PPV of ELISpot in BAL and pleural fluid for the diagnosis of TB. The results indicate the possibility of an increase of the PPV using a cut-off >1.0 for the ratio between the extra-sanguineous and systemic interferon-gamma responses. Further studies are needed to underline this ratio-approach and to evaluate full diagnostic accuracy of ELISpot in extra-sanguineous fluids like BAL and pleural fluid, as well as the impact of the results on treatment decision.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Jafari C, Ernst M, Strassburg A, et al. Local immunodiagnosis of pulmonary tuberculosis by enzyme-linked immunospot. Eur Respir J. 2008;31:261–265.
- Sester M, Sotgiu G, Lange C, et al. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J. 2011;37:100–111.
- Aggarwal AN, Agarwal R, Gupta D, et al. Interferon gamma release assays for diagnosis of pleural tuberculosis: a systematic review and meta-analysis. J Clin Microbiol. 2015;53:2451–2459.
- Hofland RW, Bossink AW, Lammers JW, et al. Pleural fluid and tuberculosis: are all interferon gamma release assays equal? J Clin Microbiol. 2016;54:504–505.
- Jafari C, Thijsen S, Sotgiu G, et al. Bronchoalveolar lavage enzyme-linked immunospot for a rapid diagnosis of tuberculosis: a Tuberculosis Network European Trialsgroup study. Am J Respir Crit Care Med. 2009;180:666–673.
- Losi M, Bossink A, Codecasa L, et al. Use of a T-cell interferon-gamma release assay for the diagnosis of tuberculous pleurisy. Eur Respir J. 2007;30:1173–1179.
- Cho OH, Park KH, Park SJ, et al. Rapid diagnosis of tuberculous peritonitis by T cell-based assays on peripheral blood and peritoneal fluid mononuclear cells. J Infect. 2011;62:462–471.
- Kim SH, Chu K, Choi SJ, et al. Diagnosis of central nervous system tuberculosis by T-cell-based assays on peripheral blood and cerebrospinal fluid mononuclear cells. Clin Vaccine Immunol. 2008;15:1356–1362.
- Lee JY, Kim SM, Park SJ, et al. A rapid and non-invasive 2-step algorithm for diagnosing tuberculous peritonitis using a T cell-based assay on peripheral blood and peritoneal fluid mononuclear cells together with peritoneal fluid adenosine deaminase. J Infect. 2015;70:356–366.
- Kubica GP, Dye WE, Cohn ML, et al. Sputum digestion and decontamination with N-acetyl-L-cysteine-sodium hydroxide for culture of mycobacteria. Am Rev Respir Dis. 1963;87:775–779.
- Savelkoul PH, Catsburg A, Mulder S, et al. Detection of Mycobacterium tuberculosis complex with real time PCR: comparison of different primer-probe sets based on the IS6110 element. J Microbiol Methods. 2006;66:177–180.
- Oxford Immunotec. 2013. Available from: http://www.oxfordimmunotec.com/international/wp-content/uploads/sites/3/PI-TB-IVD-UK-V2.pdf.
- World Health Organization. Tuberculosis. Available at: http://www.who.int/tb/en/; 2015.
- Kosters K, Nau R, Bossink A, et al. Rapid diagnosis of CNS tuberculosis by a T-cell interferon-gamma release assay on cerebrospinal fluid mononuclear cells. Infection. 2008;36:597–600.
