Abstract
Background: High-risk human papillomavirus (hr-HPV) infections and low-grade squamous intraepithelial lesions occur frequently in young women. The available vaccines cover up to seven hr-HPV genotypes (HPV16, HPV18, HPV31, HPV33, HPV45, HPV52 and HPV58) and two low-risk HPV types (HPV6 and HPV11). The objective of this study was to describe the hr-HPV genotypes present among HIV-uninfected and HIV-infected young women in rural high schools.
Methods: Cervicovaginal lavages were obtained from sexually active young women recruited from high schools in KwaZulu-Natal (n = 1223). HPV testing was done by the polymerase chain reaction using GP5+/GP6 + primers and enzyme immunoassay. HIV testing was done using rapid test kits.
Results: Of the 1223 cervicovaginal lavages, 301 (25%) were positive for hr-HPV. The HPV prevalence was higher in HIV infected (32.20%, 95% CI: 0.27–0.38) than in HIV-uninfected women (22.50%, 95% CI: 0.21–0.26), (p = .001). Similarly, multiple infections were slightly more common in HIV infected (59.32%) than in HIV-uninfected women (53.51%), (p = .37). The nine predominant genotypes in descending order were HPV types 16 (n = 99, 22.10%), 51 (n = 58, 12.91%), 18 (n = 56, 12.50%), 35 (n = 50, 11.10%), 33 (n = 47, 10.82%), 56 (n = 42, 9.31%), 45 (n = 34, 7.60%), 52 (n = 32, 7.14%) and 59 (n = 31, 6.91%). HPV 35, 51, 56 and 59 (40.62%), which are not covered by any vaccine, were among the most prevalent in the schools of KwaZulu-Natal.
Conclusion: Four of the most predominant high-risk HPV types in this region are not covered by the new nine-valent HPV vaccine.
Background
More than 100 human papillomavirus (HPV) genotypes have been identified, including over 40 types that affect the urogenital mucosa [Citation1]. The HPV genotypes have been grouped into low risk HPV (lr–HPV), probably high-risk HPV and high-risk HPV (hr-HPV) for cervical cancer [Citation2]. Nearly, 100% of cervical cancer (CaCx) cases are positive for HPV with molecular techniques [Citation3,Citation4]. About 18 mucosal HPV types are hr-HPV and persistent infections with these types increase the risk of cervical cancer [Citation3,Citation5,Citation6], some of which are strongly carcinogenic and are included in multitype vaccine to prevent cervical cancer [Citation7]. Of the 18 hr-HPV genotypes, HPV 16, 18, 45, 33, 35, 52, 51 and 31 are associated with cervical cancer in Africa [Citation8] with HPV 16, 18 and 45 reported as mostly associated with cervical cancer worldwide and in sub-Saharan Africa [Citation9,Citation10]. Twelve hr-HPV types, namely HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58 and 59, were defined as associated with cancer [Citation11]. Furthermore, HPV 16 and 18 are thought to account for approximately 70% of cervical cancers worldwide [Citation2,Citation9,Citation12]. The most prevalent high-risk type worldwide is HPV-16 [Citation9,Citation13], which shows less variation in geographical distribution compared to other types [Citation13,Citation14].
The global burden of cervical cancer is estimated at 528,000 new cases and 266,000 deaths yearly [Citation15]. The incidences and mortality rates of cervical cancer are twice to thrice lower in developed regions compared to developing countries where 86% of all cervical cancer and 88% of deaths due to cervical cancer occur [Citation16,Citation17]. According to Globocan 2012, cervical cancer is the second most common cancer among all females in less developed regions [Citation15]. In addition, cervical cancer was responsible for 25% of cancer deaths in women aged 15–44 years and 23% in those aged 45–59 years [Citation15]. In South Africa, cervical cancer was reported as a cause of death in 2% of women aged 15–44 years and in 4% of those aged 45–59 years [Citation18], a mortality rate second only to breast cancer. In addition, the cervical cancer incidence in South Africa is estimated at 31.50 cases per 100,000 people and a mortality rate of 17.90 cases per 100,000 women [Citation10]. Data from South Africa indicates that women, particularly HIV infected, have a higher risk of developing HPV-related cancers than those that are uninfected [Citation19,Citation20]. Vaccines against the common hr-HPV types 16 and 18 have been developed, which is a breakthrough in the prevention of cervical cancer [Citation21,Citation22]. Recently, a nine-valent vaccine, covering HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58, has been approved by the United States Food and Drug Administration (FDA) [Citation23]. When a new vaccine is added to a country’s immunization programme, the extent of the disease and the benefit from that vaccine should be taken into account [Citation24].
We aimed to describe the hr-HPV genotypes present in HIV-uninfected and HIV-infected young women who are beyond the age of routine hr-HPV vaccination in rural areas of the KwaZulu-Natal (KZN) province of South Africa. This epidemiological information will be beneficial in establishing the relevance of the current and proposed vaccines in the targeted population.
Materials and methods
Recruitment and enrolment
The study had a cross-sectional design and was nested in an ongoing clinical study on schistosomiasis. It was carried out in the coastal areas which have a humid and hot climate with a lot of rainfall [Citation25]. The study population consisted of young women, recruited from high schools in two rural areas on the east coast of the KZN province, referred to as Area A and Area B.
Participants were recruited for hr-HPV testing between 2010 and 2013. The study participants were interviewed by trained research assistants in their local language isiZulu, to determine the sociodemographic risks. Inclusion criteria were (1) attendance at the largest (more than 300 pupils) government high schools situated more than 10 km from the coastline and below 300 m above sea level, (2) written informed consent, (3) sexual activity, no pregnancy and above 16 years of age and (4) willingness to undergo gynaecological examination. Furthermore, at each point of the investigation, each participant was asked if she was still willing to participate.
Ethics
The clinical study was granted ethical approval from the Biomedical Research Ethics Committee of the University of KwaZulu-Natal (BF029/07). Further ethical approval was received from the Department of Health, Pietermaritzburg, KZN, (HRKM010-08; 2009), and the Regional Committee for Medical Research (REC) South Eastern Norway (Ref 469.0706Citation6aCitation1.2007.535, with amendments; Ref IRB00001870) and the European Group on Ethics in Science and New Technologies (Ref IRSES-2010:269245). In addition, ethical clearance for this study was obtained from the University of KwaZulu-Natal Ethics committee (BE234/14). All participants enrolled in the study gave written informed consent. Community members, including parents, political and traditional leaders, scholars and school leaders, were informed of the study at different meetings. HIV testing and follow-up was done in accordance with South African guidelines for pre- and post-test counselling. HIV-infected participants were offered counselling by trained counsellors and were referred to local clinics for follow-up and antiretroviral treatment. For sexually transmitted infections (STI), treatment was offered in accordance with the South African syndromic protocol by professional nurses, and when laboratory results were available, patients were treated and/or were referred to the local clinics for management. Each consenting participant underwent pre-test counselling for HIV testing before any results were conveyed. Post-test counselling was given to participants who wanted to know their HIV test results.
Clinical examination and specimen collection
Gynaecological examination was done by a female physician. Cervicovaginal lavage samples were collected by spraying 10 ml of saline on the cervix four times before drawing it back into the syringe and depositing it into sterile tubes. Specimens were kept at −80 °C until laboratory analyses were done.
DNA extraction
DNA was extracted from 200 μl of the cervicovaginal lavages using the QIAamp DNA Mini kit, (Qiagen, Whitehead Scientific, South Africa) according to the manufacturer’s instructions. The extracted DNA was kept at −80° C until analysis.
High-risk human papillomavirus detection
HPV was detected by GP5+/6 + HPV PCR [Citation26] followed by an enzyme immunoassay (EIA) method [Citation27] using a cocktail mix of 18 high-risk HPV probes. All primers and probes were obtained from Whitehead Scientific (South Africa). The polymerase chain reaction (PCR) mix consisted of 50 mM KCl, 10 mM Tris HCl buffer, 3.50 mMol MgCl2, 50 pmol GP5 + and 50 pmol GP6 + primers, 200 uM nucleotides and 5U taq polymerase (Roche, South Africa). For PCR, 10 μl of extracted DNA was added to 40 μl of PCR mix. Cycling conditions were as follows: 5 mins at 94 °C, 1 min at 94 °C, 2 mins at 40 °C, 1 min 30 s at 72 °C and 4 mins at 72 °C as described elsewhere [Citation26]. At the end of 40 cycles, the PCR products were kept at 4 °C until the EIA was performed. For EIA, streptavidin-coated plates (96-flat wells clear plates, Thermoscientific, Denmark) were used. The biotinylated GP6 + primer was captured by the streptavidin, denatured by alkaline treatment, hybridized to cocktails of digoxigenin-labelled probes, and the digoxigenin was bound by monoclonal antidigoxin alkaline phosphatase antibody (A1054, Sigma, St. Louis, MO). The wells were washed with standard saline citrate between EIA steps. A positive reaction was detected by the alkaline phosphatase colour indicator p-nitrophenyl phosphate (P4744, Sigma). The optical density (OD) was read at 405 nm using a spectrophotometer.
Six negative controls (DNA isolated from lung cancer cell line A549) and two positive controls (DNA isolated from cervical cancer cell lines HeLa and SiHa) were included in each run. The cut-off value for hr-HPV positivity of samples was calculated as the mean plus three times the standard deviation of the six negative controls in each plate. The OD of each sample (which was the average of the two wells) was divided by the cut-off value, providing a ratio. To reduce the chance of false positivity, samples with a ratio of 2 or more were regarded as HPV positive.
High-risk human papillomavirus genotyping
All samples with detected hr-HPV were further analysed to identify the genotypes present. For genotyping, individual probes were used. Individual probes for the most common hr-HPV genotypes in cervical cancer [Citation2] were selected: hr-HPV types 16; 18; 26; 31; 33; 35; 39; 45; 51; 52; 53; 56; 58; 59; 66; 68; 73 and 82. Fourteen of these were identified individually from specimens using EIA test and HPV 26 and 53 as well as HPV 73 and 82 were tested in combination. The cut-off value for hr-HPV genotype was calculated following the formula of the mean OD plus three times the standard deviation of all samples in the plate. After exclusion of outliers, the final mean +3SD was recorded and used as the cut off. This genotyping assay precludes detection of previously unknown genotypes.
HIV testing
HIV testing was done on sera using rapid test kits (SD Bioline 1/2/3.0 Rapid test kit NJ and Sensa Tri-line HIV 1/2/0, Durban, South Africa). Analyses of samples showing discrepant results were repeated.
Statistical analysis
The data were analysed using the IBM SPSS Statistics 23 package (Armonk, NY). Mean and standard deviations were used to summarize continuous variables, while proportions and frequency tables were used to summarize catergorical variables. Pearson’s chi square and Fisher’s exact tests were used to determine relationships that were interpreted using p values; p < .05 was considered as significant. To determine risk factors, bivariate analysis of sociodemographic risk factors was performed, only those with p ≤ .2 were included in the multiple logistic regression analysis. Variables found to be significantly associated with HPV infection were analysed by multiple logistic regression to find variables independently associated with HPV prevalence. The HPV genotypes that were tested in combination, namely HPV 26 + 53 and 72 + 82 (probable high risk), were excluded when the descending order of genotypes were established. Women with more than one hr-HPV type were regarded as having multiple-type infection. Hr-HPV types were counted individually as detected from hr-HPV-positive women. A new total was created out of 100% for the nine most common genotypes to establish the extent of protection by the new nine-valent vaccine against these hr-HPV genotypes.
Results
A total of 1223 women participated, and 301 (25%, 95% CI, 0.22–0.27) were hr-HPV infected. The majority of participants were between 17 and 18 years old (). The age of sexual debut ranged between 15 and 18 years (mean 16.4 years). The majority of participants reported having had one (n = 549, 45%, 95% CI, 0.54–0.61) or two sexual partners, (n = 405, 33%, 95% CI, 0.39–0.46). As shown in , there was a significant difference in both the number of lifetime sexual partners and HIV prevalence, between participants in the two areas.
Table 1. Demographic characteristics, HPV and HIV status of the participants in two areas of the KZN province.
Five variables were included in the multivariate analysis, only HIV and the presence of the father in the household were statistically significant. The presence of the father in the household was a protective factor against HPV infection (OR 0.62, 95% CI 0.42–0.93; p = .02) after correcting for confounders (). Participants who used condoms with their sexual partners were 1.19 times more likely to be HPV-infected compared to those who did not, but the difference was not statistically significant (adjusted OR 1.19, 95% CI 0.88–1.61), ().
Table 2. Risk factors associated with HPV infection among young women.
Among the 267 (22%, 95% CI, 0.19–0.24) women that were HIV infected, 86 (32%, 95% CI, 0.27–0.38) were HPV infected, (age-adjusted OR 1.82, 95% CI 1.33–2.48, p < .001). shows that hr-HPV was more common in HIV-infected participants.
Of the hr-HPV-positive lavage specimens, 166 (55%, 95% CI, 0.50–0.64) were infected with multiple hr-HPV types, whereas 135 (45%, 95% CI, 0.39–0.51) had a single HPV-type infection. Although multiple infections were more common in HIV infected (51/86, 59%, 95% CI, 0.49–0.69) than in HIV-uninfected women (115/215, 53%, 95% CI, 0.47–0.60), the difference was not significant. There was an insignificantly higher prevalence of HPV found in participants with lower age at sexual debut (OR =0.94; 95%CI: 0.69–1.29; p = .75).
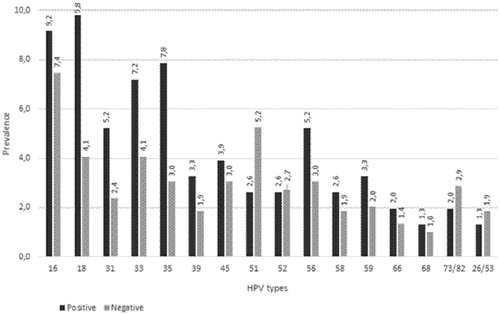
All 301 hr-HPV-positive samples were further analysed for hr-HPV-type distribution. shows the most common HPV types found in all HIV-infected and HIV-uninfected women in descending order. The nine most common HPV types found in all 301 HPV-positive women irrespective of their HIV status in descending order were HPV types 16 (n = 99, 22.10%), 51 (n = 58, 12.91%), 18 (n = 56, 12.50%), 35 (n = 50, 11.10%), 33 (n = 47, 10.82%), 56 (n = 42, 9.31%), 45 (n = 34, 7.60%), 52 (n = 32, 7.14%) and 59 (n = 31, 6.91%). HPV 35, 51, 56 and 59 (40.62%) are not covered in the new nine-valent vaccine. Unlike in , the proportions for latter most common types were calculated as a fraction of 100%. Four of the common high-risk HPV type, HPV 51, 35, 56 and 59 detected from this population are not covered by the nine-valent vaccine.
Table 3. The most predominant HPV types among young women.
The top nine most common hr-HPV types among HIV infected women were 16, 18, 35, 33, 56, 51, 45, 59 and 31. The hr-HPV types that were most common in HIV-infected women were HPV 16 and HPV 18. The nine most predominant circulating hr-HPV genotypes in descending order among HIV-uninfected women were HPV 16, 51, 18, 35, 33, 45, 52, 56, and 59. shows the most common hr-HPV types found in HIV-infected and HIV-uninfected young women. HPV 59 was among the top nine genotypes only among HIV-infected women, whereas HPV 31 was in the top nine only among HIV-uninfected young women.
Discussion
The aim of this study was to describe the predominant circulating hr-HPV genotypes among HIV-infected and HIV-uninfected young women in two areas of the KwaZulu-Natal province, South Africa. Geographical variation in the hr-HPV genotype distribution from region to region has been reported previously [Citation9,Citation14]. This study shows that the new nine-valent HPV vaccine will not protect against four of the nine most common hr-HPV types identified in our study population.
Data from 375 studies that included a total of 346 160 women in the period January 1989 to March 2007 showed a lowered or plateaued HPV prevalence with older age while other studies found a higher prevalence in older age groups [Citation28]. This meta-analysis reported a prevalence of about 20% in Africa, Central and South America while in Central and Northern Europe, Southern Europe, Middle East and Australia the recorded prevalence was 15%.
Our study is the first to report the HPV prevalence among a large sample of young women attending high school in the KZN province and describes for the first time the hr-HPV types predominant among HIV-infected and HIV-uninfected young women in KZN. An hr-HPV prevalence twice as high as the prevalence in our study (76.3%) has recently been reported among 224 women between the ages of 14 and 30 years attending a public primary health care clinic in the KZN province of South Africa [Citation29]. On the other hand, the reported prevalence from our study is similar to that of two South African studies in the Western Cape province, with participants with normal and abnormal cytology from a hospital and community health care service [Citation30,Citation31]. A community-based study in the Western Cape found a higher prevalence in participants aged 17–29 years compared to older women [Citation31]. However, in another South African primary health care-based study in the Gauteng province, a prevalence of hr-HPV of more than 50% was reported among participants aged between 16–83 years, with an even higher prevalence (66%) among those younger than 25 years [Citation32]. The latter prevalence of 66% among younger women in Gauteng province is close to that found in a similar setting in the KZN province [Citation29], which suggests that HPV prevalence is higher in young women seeking health care, although the reason for the visit is unknown.
A smaller case–control study in Gauteng province with a sample size of 159 (56 with cervical cancer and 103 with normal cytology) reported an HPV prevalence of 40% in participants with normal cytology using the sensitive Linear Array method for HPV DNA testing [Citation33]. Participants in the described study were participating in a phase-III microbiocide study and had cytological specimens taken using cytobrushes. The South African data on HPV prevalence suggests a higher prevalence in studies that are health care based and a lower prevalence in community-based investigations.
HIV infection was found in 23% of the young women in our study. This study confirmed the association between HPV infection and HIV. The HIV prevalence among the rural women in the present study concurred with that of 26% among the 15–24 years old women in rural Zimbabwe [Citation34]. However, their study had a smaller sample size of 236 compared to 1223 participants in this study. They reported a higher (34%) hr-HPV positivity in the same age group.
In Tanzania, in a cohort comprising urban and rural women, a total number of 334 out of 3603 (9.3%) women were HIV infected. In the same cohort, the prevalence of hr-HPV was 46.7% among HIV-infected women, compared to 17.2% among HIV uninfected [Citation35]. HIV is a risk factor for HPV infection that may lead to cervical, oral and anal HPV infection with the latter being most commonly associated with male homosexuals [Citation36,Citation37].
Multiple hr-HPV types occurred in more than 50% of our study population with HPV, and only slightly more frequently in HIV-infected young women. This prevalence of multitype infections is lower than that reported in other African studies which found almost twice as many multiple HPV infections in HIV-infected (64.60%) than in HIV-uninfected women (37.30%) [Citation34,Citation38].
HPV 16, 51, 18, 35, 33, 56, 52, 45 and 59 were the nine most common high-risk HPV types in young women in our study. The listed hr-HPV genotypes found in the two sampled areas are the same as those reported worldwide in a meta-analysis involving five continents [Citation39] and are similar to those reported recently for the KZN province [Citation29]. This finding is consistent with results from most geographic regions in Africa and worldwide [Citation13,Citation38].
Three types of vaccines are currently available, the bivalent (HPV 16/18), the quadrivalent (HPV 6, 11, 16 and 18) and the nine-valent vaccine (HPV 6, 11, 16, 18, 31, 33, 45, 52, 58). These vaccines were developed to prevent cervical cancer; however, HPV 6 and 11 included in the quadrivalent and nine-valent vaccine prevent genital warts. Furthermore, vaccines also prevent low- and high-grade cytological cervical abnormalities and reduces the number of infections with the HPV types covered by the vaccines [Citation40]. In South Africa, the Department of Health provides the quadrivalent vaccine in two doses to girls at the average age of 9 years (grade four learners).
The quadrivalent vaccine includes the low-risk HPV types 6 and 11 which were not analysed in this study. The quadrivalent vaccine covers only two of the top oncogenic HPV types reported in this study, whereas the new nine-valent vaccine would provide a better coverage for seven of the most common hr-HPV types. However, four of the predominant genotypes found in this study would not be covered by the new nine-valent vaccine, representing 40.6% of the top nine most common hr-HPV types in our population. The incorporation of the additional HPV types in the nonavalent vaccine provides an additional reduction of 20% in CIN1 lesions, and 30% in CIN2 and CIN3 lesions and 20% in cervical cancers, compared to the use of bi- or quadrivalent vaccines [Citation41–43].
It was noted that the presence of the father in the household was associated with lower HPV prevalence among young women. This may be attributable to the influential role of the father on the behaviour of the young woman, while the presence of the mother did not seem to impact on HPV prevalence. Although the difference was not significant, it was surprising to note from the odds ratio that participants who used condoms were more likely to have HPV infection. This may be due to inaccurate responses from young women when responding to questions on condom use.
A limitation of this study was the age of the young women enrolled (16–26 years). The presence of HPV in young women does not necessarily reflect the risk of developing cervical cancer later in life, as some types are less likely to persist, and are therefore less carcinogenic [Citation41]. Another limitation of our study was the absence of cytology results.
In conclusion, the new nine-valent HPV vaccine would not provide full protection in the KZN province of South Africa, since HPV types 35, 51, 56 and 59 are not covered in the vaccine. In addition, the results from this study may be used for the HPV vaccination follow-up research in KZN province.
Consent for publication
This manuscript contains no individual person’s data, images or videos (except for contact details for coauthors) and there are no case reports included.
| Abbreviations | ||
| hr-HPV | = | high-risk-human papillomavirus |
| DNA | = | deoxyribonucleic acid |
| OD | = | optical density |
| nm | = | nanometers |
| EIA | = | enzyme immunoassay |
| FDA | = | food and drug administration |
| PCR | = | polymerase chain reaction |
Acknowledgements
We thank the following organizations and individuals without whom this study would not have been possible:
The BRIGHT study team for support, assistance and guidance of the study participants.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- De Villiers EM. Taxonomic classification of papillomaviruses. Papillomavirus Rep. 2001;12:57–63.
- Muñoz N , Bosch FX , De Sanjosé S , et al . Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348:518–527.
- Bosch F , Lorincz A , Munoz N , et al . The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–265.
- Ferlay J , Soerjomataram I , Dikshit R , et al . GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. Lyon, France: IARC; 2013. [cited 2017 Feb 20]. Available from: http://globocan.iarc.fr
- Moscicki AB , Ellenberg JH , Farhat S , et al . Persistence of human papillomavirus infection in HIV-infected and -uninfected adolescent girls: risk factors and differences, by phylogenetic type . J Infect Dis. 2004;190:37–45.
- Moscicki AB , Ma Y , Wibbelsman C , et al . Rate of and risks for regression of cervical intraepithelial neoplasia 2 in adolescents and young women. Obstet Gynecol. 2010;116:1373.
- International Agency for Research on Cancer . 2012. Biological agents: a review of human carcinogens. Lyon, France. [cited 2017 Mar 04]. Available from: http://monographs.iarc.fr/ENG/Monographs/vol100B/mono100B.pdf
- Li N , Franceschi S , Howell , ‐et al . Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer. 2011;128:927–935.
- Clifford G , Smith J , Aguado T , et al . Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003;89:101–105.
- de Sanjose S , Quint WG , Alemany L , et al . Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048–1056.
- World Health Organization . 2014. Human Papillomavirus vaccines: WHO position paper. [cited 2017 Feb]. Available from: http://www.who.int/wer/2014/wer8943.pdf?ua=1
- Smith JS , Lindsay L , Hoots B , et al . Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007;121:621–632.
- Clifford G , Franceschi S , Diaz M , et al . HPV type-distribution in women with and without cervical neoplastic diseases. Vaccine. 2006;24:S26–S34.
- Clifford GM , Gallus S , Herrero R , et al . Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991–998.
- World Health Organization . GLOBOCAN 2012: Estimated cancer incidence, mortality and prevalence worldwide in 2012 [online]. Lyon, France: International Agency for Research on Cancer; 2014. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
- Arbyn M , Castellsague X , de Sanjose S , et al . Worldwide burden of cervical cancer in 2008. Ann Oncol. 2011;22:2675–2686.
- International Agency for Research in Cancer . Latest world cancer statistics 2013 [cited 2014]. Available from: http://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf.
- Adar J , Stevens M. Women’s health. In: Ntuli A , editor. South African Health Review 2000. Durban: Health Systems Trust; 2000. p. 411–428.
- Denny L. Human papillomavirus infection: epidemiology, clinical aspects and vaccines. TOIDJ. 2009;3:135–142.
- Ahdieh L , Klein RS , Burk R , et al . Prevalence, incidence, and type-specific persistence of human papillomavirus in human immunodeficiency virus (HIV)-positive and HIV-negative women. J Infect Dis. 2001;184:682.
- Munoz N , Bosch FX , Castellsagué X , et al . Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–285.
- Bryan JT. Developing an HPV vaccine to prevent cervical cancer and genital warts. Vaccine. 2007;25:3001–3006.
- Release FN. FDA approves Gardasil 9 for prevention of certain cancers caused by five additional types of HPV 2014. 2014. Available from: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm426485.htm.
- Bosch FX , Broker TR , Forman D , et al . Comprehensive control of human papillomavirus infections and related diseases. Vaccine. 2013;31:H1–H31.
- Rand Water . Water wise-water situation in South Africa 2015. 2015. Available from: http://www.waterwise.co.za/site/water/environment/situation.html.
- de Roda Husman AM , Walboomers JMM , van den Brule AJC , et al . The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–1062.
- Jacobs MV , Snijders P , Van Den Brule A , et al . A general primer GP5+/GP6 (+)-mediated PCR-enzyme immunoassay method for rapid detection of 14 high-risk and 6 low-risk human papillomavirus genotypes in cervical scrapings. J Clin Microbiol. 1997;35:791–795.
- Smith JS , Melendy A , Rana RK , et al . Age-specific prevalence of infection with human papillomavirus in females: a global review. J Adolesc Health. 2008;43:S5–S5.e62.
- Ebrahim S , Mndende X , Kharsany ABM , et al . High burden of human papillomavirus (HPV) ingfection among young women in KwaZulu-Natal, South Africa. PLoS One. 2016;11:10.
- Allan B , Marais DJ , Hoffman M , et al . Cervical human papillomavirus (HPV) infection in South African women: implications for HPV screening and vaccine strategies. J Clin Microbiol. 2008;46:740–742.
- McDonald AC , Denny L , Wang C , et al . Distribution of high-risk human papillomavirus genotypes among HIV-negative women with and without cervical intraepithelial neoplasia in South Africa. PLoS One. 2012;7:e44332.
- Richter K , Becker P , Horton A , et al . Age-specific prevalence of cervical human papillomavirus infection and cytological abnormalities in women in Gauteng Province, South Africa. S Afr Med J. 2013;103:313–317.
- Said HM , Ahmed K , Burnett R , et al . HPV genotypes in women with squamous intraepithelial lesions and normal cervixes participating in a community-based microbicide study in Pretoria, South Africa. J Clin Virol. 2009;44:318–321.
- Baay MFD , Kjetland EF , Ndhlovu PD , et al . Human papillomavirus in a rural community in Zimbabwe: the impact of HIV co‐infection on HPV genotype distribution. J Med Virol. 2004;73:481–485.
- Dartell M , Rasch V , Kahesa C , et al . Human papillomavirus prevalence and type distribution in 3603 HIV-positive and HIV-negative women in the general population of Tanzania: the PROTECT study. Sex Transm Dis. 2012;39:201–208.
- Beachler DC , Weber KM , Margolick JB , et al . Risk factors for oral HPV infection among a high prevalence population of HIV-positive and at-risk HIV-negative adults. Cancer Epidemiol Biomarkers Prev. 2012;21:122–133.
- Van Aar F , Mooij SH , Van Der Sande MA , et al . Anal and penile high-risk human papillomavirus prevalence in HIV-negative and HIV-infected MSM. AIDS. 2013;27:2921–2931.
- Banura C , Franceschi S , van Doorn LJ , et al . Infection with human papillomavirus and HIV among young women in Kampala, Uganda. J Infect Dis. 2008;197:555
- Bruni L , Diaz M , Castellsagué M , et al . Cervical human papillomavirus prevalence in 5 continents: meta-analysis of 1 million women with normal cytological findings. J Infect Dis. 2010;202:1789–1799.
- Garland SM , Kjaer SK , Muñoz N , et al . Impact and effectiveness of the quadrivalent human papillomavirus vaccine: a systematic review of ten years of real-world experience. Clin Infect Dis. 2016;356:ciw354.
- Guan P , Howell ‐Jones R , et al . Human papillomavirus types in 115,789 HPV-positive women: a meta-analysis from cervical infection to cancer. Int J Cancer. 2012;131:2349–2359.
- Serrano B , Alemany L , Tous S , et al . Potential impact of a nine-valent vaccine in human papillomavirus related cervical disease. Infect Agents Cancer. 2012;7:38.
- Forman D , de Martel C , Lacey CJ , et al . Global burden of human papillomavirus and related diseases. Vaccine. 2012;30:F12–F23.