Abstract
Background: Few prospective cohort studies have estimated the overall impact of severe rotavirus gastroenteritis (RVGE) leading to hospitalization on families and society. We assessed human and economic resources needed to care for an affected average child aged <5 years in Sweden.
Methods: The study was conducted in Astrid Lindgren Children’s Hospital which serves approximately 14% of all Swedish children <5 years of age. All children admitted with acute gastroenteritis in the study period were tested for rotavirus. Health care consumption was collected prospectively and publically available unit costs used to calculate direct costs. Non-medical and indirect costs were collected in interviews with families using a standardized questionnaire during the hospital stay and approximately 14 days post-discharge.
Results: 144/206 children (70%) with laboratory-confirmed RVGE were included. The median age was 14 months. The average total cost per hospitalized child was €3894, of which €2169 (56%) was due to direct healthcare-related costs (including Emergency Department visits and in-patient care), €104 (2%) to non-medical direct costs and €1621 (42%) to indirect costs due to productivity loss. Carers of children with severe RVGE were absent from work on average five days per study child: four days during hospitalization of affected child and one day due to gastroenteritis in the carer.
Conclusions: Costs for RVGE are dominated by direct costs which are similar to some other countries in Europe, but indirect costs due to productivity loss are also important, and should be considered in decisions to introduce rotavirus vaccines into national vaccination programmes.
Introduction
Infants and young children worldwide develop their first acute rotavirus gastroenteritis infection (RVGE) mostly during the first five years of life, with the great majority by the age of 2 years [Citation1]. These young children have reduced control of fluid and electrolyte balance, and may develop clinically significant dehydration requiring acute rehydration treatment in less than a day. In Sweden, children requiring nasogastric or intravenous rehydration are commonly hospitalized. Easy access to health care effectively prevents childhood deaths due to RVGE, and only occasional deaths have been reported in the last few decades [Citation2].
A decision whether to include rotavirus vaccines into the Swedish national routine paediatric immunization programme is still pending, although regional implementation in the Jonkoping and Stockholm regions was initiated in 2014, and approximately 25–30% of the Swedish birth cohort is currently offered free rotavirus vaccine as part of the routine paediatric immunization programme. Decisions on inclusion of a new vaccine into routine childhood vaccination schedules are informed by burden of disease estimates assessed in epidemiological studies, information from routine surveillance if available and cost-effectiveness assessments.
Three epidemiological studies describing the burden of severe rotavirus disease in Swedish children suggest that approximately 380–770 per 100,000 children under 5 years of age are hospitalized annually due to RVGE [Citation3–5]. Based on these incidence estimates in respective studies and an average birth cohort of approximately 110,000 newborns, we estimate that 2100–4200 children are hospitalized due to RVGE each year in Sweden.
Since rotavirus infections are not notifiable in Sweden, no reliable disease surveillance statistics on total number of children affected or hospitalized due to RVGE are available.
The total cost of extending the national vaccination programme to include rotavirus vaccines is likely to be affected by the healthcare structure and its costs, family status, degree of employment, parental allowances, vaccine cost and expected vaccine uptake in the target population. It is therefore of importance that key input variables in cost-effectiveness models are as accurate as possible. Impact studies assessing costs for affected families and society have often been neglected, and could provide data of interest to feed such models.
The aim of this study was to estimate the overall impact on families and society of RVGE leading to hospitalization before the introduction of rotavirus vaccines into the national immunization programme in a defined Swedish population. We prospectively assessed the human and economic resources needed to care for an affected child, as well as carers suffering from concomitant gastrointestinal symptoms.
Materials and methods
Study design
Impact on families and society of RVGE leading to hospitalization was assessed in a prospective cohort study conducted in 2008/2009 [Citation6,Citation7], when discussions on introducing rotavirus vaccination into the Swedish programme began. We subsequently updated the results in support of a renewed national assessment in 2016 for possible inclusion of rotavirus vaccines in 2017.
The study was coordinated by the Public Health Agency of Sweden and engaged the largest Swedish paediatric hospital; Astrid Lindgren Children’s Hospital. This public hospital serves about 66,000 children under 5 years of age (around 14% of all Swedish children of this age) living in the metropolitan area of central and northern Stockholm. No private option for in-patient care of children is available in Sweden.
Study subjects
Children under 5 years of age hospitalized overnight with community- or nosocomial-acquired acute gastroenteritis (AGE, vomiting and/or at least three loose stools/day) during the study period were tested for excretion of rotaviruses. All children with laboratory-confirmed RVGE were documented and their families were asked for availability for interview by a designated study nurse. The nurse was available 5 days per week (Monday through Friday). Informed consent was obtained from carers willing to participate in the study. Reasons for not wanting to participate were documented. All data were anonymized before the analysis. Privacy rights of included subjects and their families were observed.
Rotavirus vaccines were available before and during the study period in the private market but there is limited tradition among Swedish families to vaccinate over and above the nationally offered free vaccines, despite of occasional available subsidies.
Rotavirus diagnosis
Presence of rotavirus in faecal samples was assessed by either a rapid test (Vikia™, Biomerieux, France) or ELISA (IDEA™ Rotavirus, DakoCytomation Ltd, UK, cut-off 0.7 OD). Sensitivity and specificity of the VIKIA Rota-Adeno immuno-chromatographic test and the ELISA IDEIA Rotavirus kit were 96.6% (95% confidence intervals [CI] 83.0; 99.9) and 96.4% (95% CI 81.6; 99.9), respectively [Citation8]. All rotavirus-positive samples in rapid tests were confirmed by an in-house PCR at the Swedish Public Health Agency using methodology previously described [Citation4].
Carer interviews
The first interview was conducted with consenting families during the hospital stay, and a follow-up interview was performed approximately two weeks after discharge. During both interviews, standardized questionnaires requested information on age and sex of the affected child, age of both parents, socioeconomic parameters such as educational level of carers, whether either of the carers were receiving parental allowances or worked part/fulltime at the time of admission of the affected child, whether any other household member developed gastrointestinal symptoms within 14 days from onset of disease in the hospitalized child, number of working days lost by carers due to gastrointestinal symptoms in the hospitalized child or another household member, and utilization of health care and consumables. Based on collected information costs for healthcare accrued before, during and after hospitalization, non-medical services and productivity loss were calculated per study child.
Calculation of costs
Healthcare-related costs paid by the society were calculated by multiplying consumption with unit costs using standardized Swedish national price lists for visits to primary care, Emergency Department (ED) visits or in-patient stay at hospital (diagnosis-related group (DRG) for hospital stay due to acute viral gastroenteritis or RVGE used by the hospital) (). This means that study driven costs such as testing for excretion of rotaviruses were not included. For hospitalized children treated in intensive care, an additional cost was added. We calculated mean resource consumption for all participating families.
Table 1. Unit costs and source.
We used Swedish unit costs (SEK) for year 2015 from price indexes for county councils. Results were converted from SEK to EURO (€) using the average exchange rate in 2015 (9.36). The costs of consumables paid for by families (for example, pharmaceuticals, oral rehydration solution, nutritional supplements) and non-medical services were reported directly by the carers. Private transportation costs were calculated from estimated mileage as reported by carers at €0.2/km.
Costs due to productivity loss (full-time equivalent) accrued by different household members before, during and after hospitalization of an affected child, were calculated (). A sub-analysis considered days of work lost for families in which two carers worked full-time.
Ethical permission
The study was approved by the Karolinska Institute Ethics Committee (Dnr 2007/578-31/4) and was conducted according to Good Clinical Practice and the 1996 version of the Declaration of Helsinki.
Statistics
SPSS (IBM Corp. Released 2015. IBM SPSS Statistics for Windows, Version 23.0. Armonk, NY, USA)AQ2 was used for data analysis. Cost and visit data are shown as mean of all children, standard deviation (SD) and 95% CI. Age is expressed as median and interquartile range (IQR). Categorical variables are expressed as number and percentage (n [%]).
Results
Number of families of study children available for interview
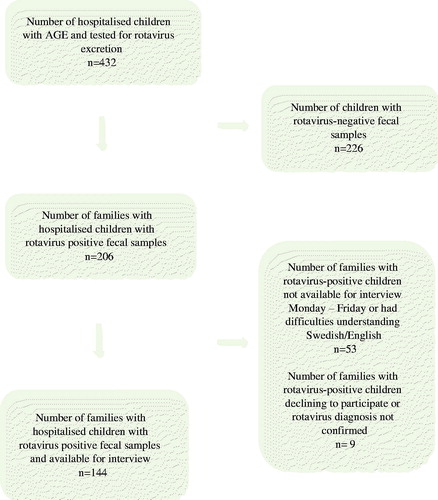
Of 432 children with AGE admitted during the study period, 206 children (48%) were identified with laboratory-confirmed rotavirus infection and families of 144 rotavirus-positive children (70%) were available for interview. Most children (89%) acquired their rotavirus infection in the community, while 11% of the children developed their infection following nosocomial transmission. Reasons for families not participating were admission during weekends, difficulties in understanding Swedish or English, declined participation or the diagnosis of rotavirus could not be confirmed by PCR (). None of the included children had received any rotavirus vaccine.
Age and gender of study children
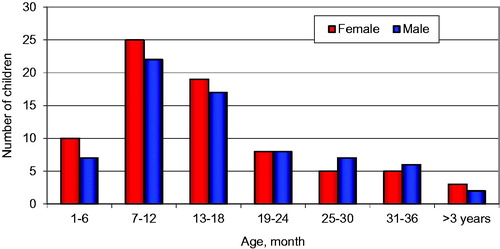
Of the 144 included study children, 75 (52%) were girls and 69 (48%) were boys. The median age of all children was 14 months (mean 16.3 months); 13 months in girls (mean 15.9. range 1–55 months) and 14 months in boys (mean 16.8, range 1–44 months) ().
Socioeconomic parameters characterizing carers
In this material, the formal carer of the affected child while hospitalized was one of the child’s parents. The median age of mothers and fathers was 33.0 and 35.5 years, respectively. After discharge from hospital, other carers occasionally continued treatment in the home setting ().
Table 2. Socioeconomic parameters of parents to hospitalized children with laboratory confirmed rotavirus gastroenteritis (n = 144).
The educational level of parents was overall relatively high in this urban area; 65% of all mothers (94/144) and 62% of all fathers (85/138) had a university degree (). This is higher than the overall educational level in the country at the time of the study; in which 39% of females and 31% of males in this age group reported a university degree [Citation9].
At the time of hospital admission, 33/144 (23%) of mothers worked full-time, 24/144 (17%) worked part-time and 86/144 (60%) received parental leave allowance from the Swedish government. Of the 138 fathers, 114 (83%) worked full-time, 11 (8%) worked part-time and 10 (7%) received parental leave allowance from the Swedish government ().
Costs for health care accrued before hospitalization of study child
There were 26/144 families (18%) that visited a primary care facility prior to hospitalization for the symptoms of gastroenteritis, and that were part of the rotavirus episode requiring hospitalization. A further 38/144 (26%) visited an ED facility (in addition to the ED visit leading to hospitalization), 104 (72%) had a telephone contact with a paediatric nurse and one received a home visit by a paediatric nurse. The average cost for visits before hospitalization per study child was €145, including cost of drugs, oral rehydration solution and nutritional supplements ().
Table 3. Utilization of health care, consumption of non-medical services and calculated average cost per child in the whole study (Euros (€) 2015).
In total, 49% (70/144) of all families purchased any type of pharmaceuticals before hospitalization, 64% (92/144) purchased oral rehydration solution and 25% (36/144) purchased a nutritional supplement (commonly blueberry or carrot soup/puree). The average total cost for drugs, oral rehydration solution and nutritional supplements per child, before hospitalization was €13 ().
Costs for healthcare accrued during hospitalization of study child
All study children were admitted to a paediatric infectious disease ward. In addition, two children required intensive care for one and three days, respectively. The average cost for hospitalization per child in the study was approximately €1998, irrespective of length of hospital stay ().
Costs for healthcare accrued after discharge of study child
Following discharge 12/144 (8%) children were seen by a doctor for clinical evaluation. The average cost per child was €15, excluding cost for additional drugs, oral rehydration solution or nutritional supplements ().
There were 15/144 families (10%) that purchased any type of drug after hospitalization, 18% (26/144) purchased oral rehydration solution and 38% (55/144) purchased a nutritional supplement. The average cost per child was approximately €25 ().
Costs of non-medical services accrued by families
Six of 144 families (4%) used external care of the sick child during the AGE episode, at an average cost per child of €8. Other costs for non-medical services included transportation and other consumables, to an average cost of €104 per child ().
Loss of working days for carers due to care for study child
In total 49/144 (34%) mothers reported lost work days due to care of the study child. Mean working days lost amongst those mothers was 4.6 days per hospitalized child, equivalent to 1.6 days per child across the whole study. In total, 98/138 (71%) of fathers (representing 68% of families [98/144]) reported loss of working days due to care for study child. Mean working days lost among those fathers was 3.3 days per hospitalized child, equivalent to 2.3 days per child across the whole study. In addition, following discharge, 24 other routine carers such as grandparents reported loss of working days due to care for the study child. Mean working days lost by ancillary carers was 2.2 days, equivalent to 0.4 days per average study child ().
Table 4. Working days lost by carers due to care of child or their own gastrointestinal symptoms: all families and families where both parents work full-time.
A sub-analysis of lost work days was conducted for 23 families with two carers working full-time (). In these 23 families, 22 mothers reported a mean of 4.9 lost work days per hospitalized child, and 20 fathers reported a mean of 3.5 lost work days per hospitalized child. The mean number of work days lost for these families was 8.2.
Loss of working days for carers due to own gastrointestinal symptoms
There were five mothers, 12 fathers and 30 other carers who reported work days lost due to own gastrointestinal symptoms, equivalent to 1.2 days per child across the whole study ().
Costs accrued by absence from work
There was a large proportion of carers (118/144 families, 82%) who reported absence from work to care for a sick child. The average number of work days lost per study child was 5.4 days per family, of which 4.2 days were due to care of sick child, and 1.2 days were due to sick leave by a symptomatic carer (). The total productivity loss was 776 full-time days. Assuming that a value of production loss was about €300 per full-time working day in Sweden 2015 [Citation10] the cost was slightly more than €1621 per child on average ().
Overall distribution of costs
The estimated average total cost per hospitalized child due to RVGE was €3894, of which €2169 (56%) was due to direct healthcare related costs (including ED visits and in-patient care), €104 (3%) to non-medical direct costs, and €1621 (42%) to indirect costs due to productivity loss.
Discussion
Impact studies assessing costs for society and affected families have often been neglected, but are of particular interest to feed cost-effectiveness models conducted to inform decisions on whether to include rotavirus vaccination in national immunization programmes [Citation11–21]. Impact studies could contribute to reduce uncertainty in import variables and increase the validity of modelled results.
In this study of the cost impact of hospitalized children with laboratory-confirmed RVGE in Sweden on families and society, we observed that the economic burden is primarily driven by costs related to in-patient care, which is sensitive to the unit cost used. The direct cost of 2169€ per RVGE admission in Sweden is higher than in Belgium, England and Wales, Finland and France, but similar to that in the Netherlands [Citation22]. In addition, costs for loss in productivity among parents and carers in our study is substantial, 42%, despite generous parental allowances paid by the Swedish government. In Sweden, families are entitled to 12–18 months (full-time 12 months or part-time 18 months) economic full- or part-time support for each child.
A recent cost-effectiveness analysis of the possible introduction of rotavirus vaccination into the Swedish childhood immunization program shows the importance of considering the impact of indirect costs [Citation21]. In this study, an incremental cost-effectiveness ratio (ICER) of 60,000€ per quality adjusted life years (QALY) gained was calculated using only direct costs. Our study suggests that the ICER for rotavirus vaccination in Sweden may be lower than previously calculated, confirming that the inclusion of indirect costs in the base case analysis would likely result in significant health gains at a lower cost for society.
Paid parental leave for either parent was introduced in Sweden in 1974. Adjustments to this system have been made repeatedly, and since 2002 a family is entitled to 480 days of parental leave in total, to be taken from when the child is between 0 and 8 years of age. The 480 days (90 days with limited pay of €18 per day and the rest dependent upon salary before childbirth) can be used at any age, but most of families choose to use them first two years of life. The system provides flexibility as to when, how, and to what extent either parent returns to work. Three months of the parental leave is reserved for fathers. In 1974, 0.5% of all Swedish men chose to use this offer, increasing to >22% when our study was conducted. For a disease like acute RVGE which mainly affects children younger than three years of age, estimating the number of days of work-loss needs to be assessed through interview, because no family chooses the same solution for childcare in general, and in particular when a child is ill. The proportion of Swedish children in day care at the age of 12 months is 48.9%, at 24 months is 91.3% and at 36 months is 94.8% according to statistics developed by the Swedish National Agency for Education [Citation23].
An earlier study (REVEAL) which assessed costs for RVGE in seven European countries, including Sweden, showed that costs, particularly indirect costs, varied substantially between study countries (e.g. workdays lost ranged from 2.3 to 6.4 days for children requiring hospitalization) [Citation24]. Swedish data in the REVEAL study were derived in a study centre in Västerbotten County in northern Sweden, and showed that the number of working days lost due to care of a sick child requiring hospitalization was 4.0 days, much in line with the 4.2 days in our study. Our study also identified that another 1.2 working days were lost due to sick leave incurred by gastroenteritis in the carer. Moreover, Swedish data in the REVEAL study showed that for children in need of hospitalization, direct medical costs represented 72% of the total cost versus 52% in our study, direct non-medical costs for services represented 1% versus 3% in our study, and indirect costs due to working days lost represented 27% versus 42% in our study. The higher proportion of indirect costs in our study can partly be explained by the extra day of working loss identified due to illness in the carer, but may also be explained by differences in setting, time of data collection and basis of calculation. A limited number of studies have been conducted to study transmission of rotaviruses to family members of children with RVGE, and to assess the costs of hospitalized children with RVGE. Senecal et al. [Citation25] suggested that in Canada at least one other family member developed gastrointestinal symptoms in almost half of investigated households, possibly leading to school absence in older children or work loss in carers. Similarly, in UK families another family member developed gastroenteritis in 52% of RVGE cases [Citation26]. While secondary cases are not often included in the calculation of total cost, we consider that these cases are preventable through vaccination of children, and represent a valid cost component of RVGE. Jacobs et al. [Citation27] showed that a child in Canada was typically hospitalized for approximately 2.5 days, leading to a cost (direct and indirect) of approximately €2000 per child.
The largest total costs of rotavirus in society are those associated with severe disease requiring hospitalization, and national immunization technical advisory groups are often most interested in the ability of the vaccine to prevent this outcome. Accordingly, the focus of our study was on severe disease. We estimate that hospitalization of at least 2100 RVGE cases per year in Sweden leads to a cost of €7.5 million. However, many or even most children with RVGE are seen only in outpatient settings, although these milder illnesses may also cause absence of family members from work. In the REVEAL study, costs for RVGE treated in an outpatient setting were lower compared to costs for children requiring in-patient care, but no data were provided on the proportion of RVGE patients who were only treated in an outpatient setting and the cost burden of this population in Sweden remains unknown [Citation28].
In spite of recommendations from the World Health Organization issued as far back as 2007 (updated in 2009) [Citation29,Citation30], many European countries have not introduced rotavirus vaccines ten years after the authorization of the second generation of rotavirus vaccines. Cost of vaccination has been viewed as a major hurdle to the introduction of these vaccines into the national programmes in several countries [Citation31]. As of 2016, 12 EU/EEA countries (Austria, Belgium, Estonia, Finland, Germany, Greece, Ireland, Latvia, Luxembourg, Norway, Poland and the UK) have existing recommendations for universal rotavirus vaccines in their national paediatric immunization programmes. Italy and Sweden have implemented rotavirus vaccination in parts of the country.
This is one of few studies that assess the total societal impact of hospitalized RVGE. One of the strengths of our study is that we used documented reimbursement costs of the Swedish health care system for children with severe rotavirus diarrhoea. While there is some variation in the DRG reimbursements throughout Sweden, this is limited to approximately 3–4%. Therefore, our results are likely to be representative for direct medical costs across Sweden. Given the setting-specific parental leave allowances and patterns of day care use in Sweden, we conducted a sub-analysis of work days lost in families with both parents working. These results could be extrapolated to settings outside of Sweden with different patterns of parental work and childcare, making our study more widely applicable.
Potential limitations of the study are that recruitment of families only occurred Monday through Friday in the mornings, and from admissions that happened overnight. Although we have no reason to believe that these RVGE cases differ from children admitted during weekends, selection bias cannot be excluded. Further, the sample size is limited even though the hospital serves approximately 66,000 children <5 years of age in the metropolitan and urban areas of the northern Stockholm country, which is equivalent to 14% of the Swedish paediatric population in this age group. Average time for inpatient treatment due to acute gastroenteritis varies significantly across the country (range 1–3 days), but since all hospitals receive reimbursement according to standard pre-agreements according to the DRG system, this does not influence the costs calculated in our study. In a previous Swedish study the median total duration of diarrhoea was shown to be 6.9 days, which likely is the reason in addition to their own illness why carers had to be absent from work [Citation4]. Finally, the educational level of parents was higher than the overall educational level in the country at the time of the study which may have influenced employment and income levels, and healthcare service utilization. Any impact on our findings is uncertain, but the use of National Labour Cost Survey data to estimate lost wages mitigates potential differences between income level between the study group and the general Swedish population.
Conclusions
Severe rotavirus infections leading to hospitalization have a significant economic impact on affected families and Swedish society. Costs for health care dominate, but indirect costs due to productivity loss are also an important cost, and should be considered in the decision-making process whether to introduce rotavirus vaccines into national vaccination programmes.
Acknowledgements
We wish to thank all families devoting their time to answer questions in the case report form. We also thank Bo Östlund at the Swedish Public Health Institute for project management and Elisabet Nicolic at the Center for Medical Technology Assessment, Department of Medicine and Health, Linköping University for data support.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Parashar U, Hummelman E, Bresee J, et al. Global illness and deaths caused by rotavirus disease in children. Emerging Infect Dis. 2003;9:565–572.
- Johansen K, Hedlund KO, Zweygberg-Wirgart B, et al. Complications attributable to rotavirus-induced diarrhoea in a Swedish paediatric population: report from an 11-year surveillance. Scand J Infect Dis. 2008;40:958–964.
- Johansen K, Bennet R, Bondesson K, et al. Incidence and estimates of the disease burden of rotavirus in Sweden. Acta Paediatr Suppl. 1999;88:20–23.
- Rinder M, Tran AN, Bennet R, et al. Burden of severe rotavirus disease leading to hospitalization assessed in a prospective cohort study in Sweden. Scand J Infect Dis. 2014;46:294–302.
- Giaquinto C, van Damme P. Age distribution of paediatric rotavirus gastroenteritis cases in Europe: the REVEAL study. Scand J Infect Dis. 2010;42:142–147.
- Tran A, Bennet R, Cassel T, et al. Transmission of rotavirus to household members of Swedish children hospitalized for severe rotavirus gastroenteritis results in loss of parental productivity. 27th Annual Meeting of the European Society for Paediatric Infectious Diseases, ESPID Brussels, Belgium, June 9–13, 2009.
- Johansen K, Brytting M, Husberg M, et al. Estimates of healthcare and non-healthcare costs due to severe rotavirus infections leading to hospitalization in Swedish children (<5 years). 29th Annual Meeting of the European Society for Paediatrics Infectous Diseases, The Hague, 7–11, 2011.
- de Rougemont A, Kaplon J, Billaud G, et al. Sensitivity and specificity of the VIKIA Rota-Adeno immuno-chromatographic test (bioMérieux) and the ELISA IDEIA Rotavirus kit (Dako) compared to genotyping. Pathol Biol (Paris). 2009;57:86–89.
- Statistics Sweden. SCB Utbildningsstatistisk årsbok. Available at http://www.scb.se/en_/ 2010
- Rappuoli R, Aderem A. A 2020 vision for vaccines against HIV, tuberculosis and malaria. Nature. 2011;473:463–469.
- Bilcke J, Van Damme P, De Smet F, et al. The health and economic burden of rotavirus disease in Belgium. Eur J Pediatr. 2008;167:1409–1419.
- Jit M, Edmunds WJ. Evaluating rotavirus vaccination in England and Wales. Part II. The potential cost-effectiveness of vaccination. Vaccine. 2007;25:3971–3979.
- Lorgelly PK, Joshi D, Gomara MI, et al. Exploring the cost effectiveness of an immunization programme for rotavirus gastroenteritis in the United Kingdom (Structured abstract). Epidemiol Infect. 2008;136:44–55.
- Aidelsburger P, Grabein K, Böhm K, et al. Cost-effectiveness of childhood rotavirus vaccination in Germany. Vaccine. 2014;32:1964–1974.
- Goossens LM, Standaert B, Hartwig N, et al. The cost-utility of rotavirus vaccination with Rotarix (RIX4414) in the Netherlands. Vaccine. 2008;26:1118–1127.
- Zomer TP, van Duynhoven YTHP, Mangen MJJ, et al. Assessing the introduction of universal rotavirus vaccination in the Netherlands. Vaccine. 2008;26:3757–3764.
- Mangen MJ, Duynhoven YT, Vennema H, et al. Is it cost-effective to introduce rotavirus vaccination in the Dutch national immunization program? (Structured abstract). Vaccine. 2010;28:2624–2635.
- Giammanco MD, Coniglio MA, Pignato S, et al. An economic analysis of rotavirus vaccination in Italy (Structured abstract). Vaccine. 2009;27:3904–3911.
- Tilson L, Jit M, Schmitz S, et al. Cost-effectiveness of universal rotavirus vaccination in reducing rotavirus gastroenteritis in Ireland. Vaccine. 2011;29:7463–7473.
- Imaz I, Rubio B, Cornejo AM, et al. Budget impact and cost-utility analysis of universal infant rotavirus vaccination in Spain. Prev Med. 2014;61:116–121.
- Folkhälsomyndigheten. Hälsoekonomiskt kunskapsunderlag – Rotavirusvaccination En kostnadseffektivitetsanalys av ett införande av rotavirusvaccination i det svenska barnvaccinationsprogrammet. 2015.
- Jit M, Bilcke J, Mangen MJ, et al. The cost-effectiveness of rotavirus vaccination: comparative analyses for five European countries and transferability in Europe. Vaccine. 2009;27:6121–6128.
- Swedish National Agency for Education. Skolverket. Available from: https://www.skolverket.se/om-skolverket/andra-sprak/in-english.
- Giaquinto C, Van Damme P, Huet F, et al. Costs of community-acquired pediatric rotavirus gastroenteritis in 7 European countries: the REVEAL study. J Infect Dis. 2007;195:S36–S44.
- Senecal M, Brisson M, Lebel MH, et al. Measuring the Impact of Rotavirus Acute Gastroenteritis Episodes (MIRAGE): a prospective community-based study. Can J Infect Dis Med Microbiol. 2008;19:397–404.
- Marlow R, Finn A, Trotter C. Quality of life impacts from rotavirus gastroenteritis on children and their families in the UK. Vaccine. 2015;33:5212–5216.
- Jacobs P, Shane LG, Fassbender K, et al. Economic analysis of rotavirus-associated diarrhea in the metropolitan Toronto and Peel regions of Ontario. Can J Infect Dis. 2002;13:167–174.
- Bergman A, Young C, Maidi-Fargier H, et al. Health care and society have to pay a high price for rotavirus infections in children. A Swedish descriptive cost analysis study. Lakartidningen. 2008;105:1186–1191.
- Meeting of the WHO Strategic Advisory Group of Experts on immunization A–car. Position paper rotavirus vaccines. Wkly Epidemiol Rec. 2007;82:285–295.
- Meeting of the WHO Strategic Advisory Group of Experts on immunization A–car. Position paper rotavirus vaccines. Wkly Epidemiol Rec. 2009;84:220–236.
- Parez N, Giaquinto C, Du Roure C, et al. Rotavirus vaccination in Europe: drivers and barriers. Lancet Infect Dis. 2014;14:416–425.
- Rahmqvist M, Husberg M. Effekter av sjukvårdsrådgivning per telefon: en analys av rådgivningsverksamheten 1177 i Östergötland och Jämtland. CMT Rapport. 2009;3.
- 1177 Vårdguiden. Available from: http://www.1177.se/Stockholm/Regler-och-rattigheter/Sjukresor-i-Stockholms-lan