Abstract
Background: Colonization with methicillin-resistant Staphylococcus aureus (MRSA) can cause endogenously derived infections and be a source of transmission to other people. Neither colonization time of asymptomatic MRSA colonization nor the effect of treatment aiming at MRSA eradication in children has been thoroughly investigated.
Methods: Two hundred ninety-three children <18 years in the mandatory follow-up program for MRSA-carriers in Malmö, Sweden were evaluated. Samples from the throat, nares, perineum and skin lesions from each child were screened for MRSA with a PCR-based broth enrichment method. PVL presence and spa-type were evaluated in a majority of cases. The sampling was repeated approximately every 6 month after initial detection. When three consecutive sets of negative samples during at least a 6-month period were obtained, the MRSA was considered permanently eradicated. MRSA eradication treatment given, on clinical grounds during follow-up, was noted.
Results: One year after detection 62% of the untreated children were still MRSA positive and after 2 years 28%. MRSA throat colonization and having MRSA positive household contacts significantly prolonged the observed colonization time. Topical MRSA eradication treatment was successful in 36% of cases and in 65% if systemic antibiotics were added. Presence of PVL correlated with shorter observed colonization time in the older age group and with increased eradication success among throat carriers.
Conclusion: MRSA throat colonization and having MRSA positive household contacts prolongs the time of MRSA colonization in children. Systemic antibiotics enhance the effect of MRSA eradication treatment.
Introduction
Staphylococcus aureus causes considerable disease burden both by nosocomial and community-acquired infections. Globally and in Sweden, the methicillin-resistant S. aureus (MRSA) prevalence has increased in recent decades, resulting in higher S. aureus morbidity, mortality and increased healthcare costs [Citation1–4]. According to the Swedish Communicable Diseases Act, registration and follow-up of MRSA colonization as well as contact tracing has been mandatory in Sweden since January 2000. In combination with low antibiotic prescription rates, this has probably contributed to the low Swedish MRSA prevalence of <3%, in both S. aureus blood isolates and in samples from skin and soft-tissue infections (SSTI) [Citation1,Citation5].
World-wide, as well in Sweden, MRSA used to be healthcare associated, but the community-acquired MRSA cases are increasingly affecting healthy individuals, especially children and young adults [Citation1,Citation6,Citation7].
Asymptomatic colonization can cause endogenously derived infections [Citation8–10] and be a source of transmission to other individuals. The colonization time varies in different studies with different settings [Citation11–17]. Especially among children, the colonization time is not well explored, although some studies have shown that colonization varies with age [Citation13,Citation18] and the frequency of S. aureus nasal colonization is demonstrated to decrease with higher age [Citation8].
General risk factors described for prolonged MRSA colonization are skin lesions, colonization of two or more body sites, low age and infection prone clones [Citation11–13]. Nasal decolonisation of MRSA is described to diminish the progress to manifest infections [Citation19]. Systemic antibiotic treatment in the attempt to eradicate MRSA in special patient categories, such as those with cystic fibrosis with pulmonary carriage of MRSA, has been demonstrated with an approximate success rate of 54% [Citation20]. Dermatological patients have also been studied, and topical treatment seems efficient in most cases [Citation21].
The effect of systemic treatment in a general population carrying MRSA has been evaluated in the Netherlands with a success rate of 60% [Citation22], but the effect in different age groups has not been fully evaluated.
In the present study, observed MRSA colonization time and the effect of eradication treatment have been investigated among children under 18 years.
Material and methods
Setting
The study was performed at the outpatient section of the Malmö division of the Infectious Disease Department at the tertiary care Skåne University Hospital. The department is the only infectious disease facility in a geographical area with 380,000 inhabitants, located in the south of Sweden.
Study design
All MRSA carriers under 18 years, detected in the catchment area between the years 2001 and 2012, were included. Initial cultures were obtained either from a clinical infection, by screening or from contact tracing. Mandatory screening was performed according to regional regulations in all individuals admitted to the hospital who had received inpatient hospital care abroad for >24 h during the last 6 months. Contact tracing was performed according to national regulations in all household members within 2 weeks of detection of the first MRSA positive individual in the household. All MRSA positive members of a household were included as separate cases. MRSA positive individuals were notified according to the Swedish Communicable Diseases Act and included in a local mandatory MRSA follow-up register. When detected from a clinical infection, MRSA cultures were performed from the anterior nares, throat, perineum and skin lesions within 2 weeks to explore the degree of colonization. Children treated with effective antibiotics after one initial MRSA positive culture from an acute SSTI, were not considered MRSA carriers nor included in the study, if MRSA could not be detected in the wound or at any other body site during follow-up. Children with only one initial MRSA positive nasal culture, where all subsequent follow-up cultures turned out negative, were considered transient carriers and not included in the study.
Pregnant women were screened for MRSA equivalent to regulations for patients who were to be admitted to hospital. Children born by a MRSA positive mother were consequently screened as contact tracing.
After initial detection, MRSA cultures were obtained approximately every 6 month from the mentioned body sites and from any other body site previously found to be MRSA positive. Children who had failed to come for their planned culture were excluded and considered lost-to follow-up if >12 months had passed between a positive culture and a negative culture set. If MRSA was detected in the following set of cultures and presented the same antibiogram, the child was considered still positive, even though >12 months had passed between the cultures. When eradication treatment was given, a first set of cultures were obtained 2 weeks after the end of treatment. If these cultures turned out negative they were followed by cultures every 2–3 months. In children with spontaneously obtained negative cultures, new sets of culture were also obtained every 2–3 months. When three consecutive negative sets of samples were obtained within at least 6 months, the child was declared MRSA negative and the mandatory follow-up was ended. Before 2008, an additional culture was obtained 6 months later, but this regulation was ended due to evaluations [Citation13] showing that it did not add more information even in persons with skin lesions. In children declared MRSA negative, the observed colonization time was calculated as the time period between the first MRSA positive culture and the first MRSA negative culture set. Children still MRSA positive when the study period was ended in April 2017 were included in the study, and the observed colonization time was calculated as the time period between the first obtained MRSA positive culture and the most recent culture set taken. Information about antibiotic treatment, degree and observed time of MRSA colonization and MRSA status of household members were retrieved from the patient chart and the local MRSA follow-up register.
Samples
During the study period, two different types of swabs were used. During 2001–2012 rayon tipped swabs with agar gel Amies transport medium with charcoal (Copan Italia, Brescia, Italy) and since 2013 Copan E-swabs (Copan Italia) were utilized. Samples were taken from nostrils, throat and perineum. If skin lesions were present, they were also sampled after cleaning. E-swabs were stirred for 30 s in the transport media which was forwarded without the swab to the Clinical Microbiology Laboratory in Malmö.
MRSA screening and detection
Traditional agar-plate based MRSA screening was replaced by an enrichment broth method in 2003 [Citation23]. From 2008, methicillin was replaced with cefoxitin and aztreonam for more stable enrichment [Citation24]. For E-swab transport media, 100 µL of the medium was used to inoculate the enrichment broths.
Between 2003 and 2010, 100 µL of each enrichment broth per sample was pooled, using maximum three samples per pool. A crude DNA extract was made from each pool [Citation23]. Two µL of the DNA extract was used as a template for a quantitative PCR amplification of Sa442 [Citation25]. Enrichment broths from pools containing >10,000 genome equivalents of S. aureus per mL were plated individually onto selective and non-selective agar plates with a 10 µg cefoxitin disk on the non-selective plate. Phenotypically identified S. aureus with reduced susceptibility to cefoxitin were checked for the presence of mecA-gene [Citation26] and verified as S. aureus with PCR against the nuc-gene [Citation27].
In 2011, a new DNA extraction and multiplex amplification technique (see supplementary materials and methods and supplementary table 1) was introduced and pooling of enrichment broths was discontinued.
All new MRSA isolates were susceptibility tested according to the national guidelines utilizing EUCAST breakpoints. Susceptibility testing was repeated if >3 months had passed since last testing and after eradication attempts.
Molecular characterization by spa-typing and detection of the genes encoding Panton-Valentine leukocidin (PVL), lukS-PV and lukF-PV was performed as described elsewhere [Citation28].
Eradication treatment
Treatment attempting to eradicate MRSA was given simultaneously to all MRSA positive household members if any member in the household had repeated infectious problems or an ongoing MRSA infection requiring treatment, expected frequent healthcare contacts, health care work or if any form of social reasons existed (mostly strong wishes to attempt eradication by treatment). None of the children in the followed untreated group had a household member receiving eradication treatment since all members in a household were treated simultaneously. Information about antibiotic treatment initiated in the primary care was not available in this evaluation. The practice used in the area was, however, that MRSA carriers with SSTIs were referred to the infectious disease department for treatment but respiratory or urinary tract infections could be treated in the primary care mostly with MRSA inactive antibiotics. The eradication treatment was standardized except regarding the type of antibiotics used. The choice to treat, and antibiotics used, was made on the discretion of the physician in charge. Topical treatment consisting of 7 days nasal mupirocin three times daily and a 14 day hygiene program, including showering with chlorhexidine and the change of clothing and bed linen at least twice a week, was used. For the great majority of treated children who carried MRSA in the throat systemic antibiotic treatment for 14 days was included in the treatment regime. The antibiotics used in nearly all the cases was rifampicin (if susceptible) in combination with one other drug the strain was susceptible to; clindamycin, fucidic acid or trimethoprim/sulfamethoxazole.
Statistical methods
Proportions of categorical variables were analyzed with Chi-squared test, or Fishers exact test when expected numbers approximated <5. The continuous variables age and duration of colonization were not normally distributed and univariate comparisons were therefore analyzed with non-parametric Wilcoxon rank-sum test (Mann–Whitney U-test), and log-rank test, respectively, while multivariate comparison of colonization time was performed with Cox proportional Hazards regression. Proportional hazards assumption was controlled graphically and with testing of Schoenfeld residuals. Multivariate analysis of eradication strategy was performed using logistic regression. All analyses were performed using Stata 14.0 (Statacorp, College Station, TX).
Ethical considerations
The study was approved by the Lund University ethical committee (Dnr 2015/48) who imposed publishing of an advertisement in the daily press informing the public on the study with an opt-out possibility. Personal information about the study was not possible due to frequent changes of addresses in the study group, and the addresses were not available once an individual had been declared MRSA negative.
Results
During the period 2001–2012, 368 MRSA positive children were detected in the area. Out of those 75 were excluded (lost to follow-up, lack of data, transient carriership or initial MRSA SSTI given antibiotic treatment). The remaining study group consisted of 293 children. In 96 of these children treatment was given aiming at MRSA eradication. A flow chart is presented in . Cases declared lost to follow-up were mainly disqualified due to >1 year lag time between last positive and first negative culture or because of emigration from the area. Baseline data are shown in .
Figure 1. Flow chart displaying inclusion and exclusion of the MRSA positive children in the study. aExcluded due to transient carriership or insufficient data. bChildren with MRSA SSTI given antibiotic treatment, where no other initial or later cultures indicated MRSA colonization.
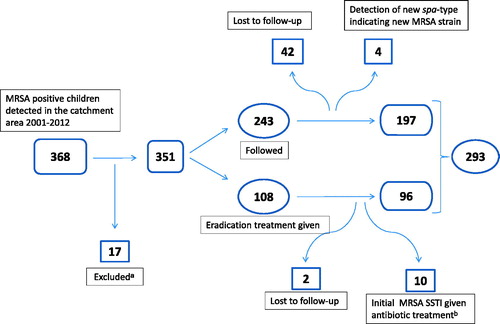
Table 1. Baseline characteristics of the children included in the study.
Observed time of colonization
Children who did not receive MRSA eradication treatment cleared MRSA by time and the number of affected children decreased gradually. One year after detection of MRSA colonization 62% (122/197) were still positive and after 2 years 28% (55/197) remained positive (). The median time of colonization after detection was 14.9 months (interquartile range [IQR] 7.0–25.0) in the 197 followed children not given eradication treatment. Factors significantly prolonging the observed time of colonization were throat colonization and having MRSA positive household contacts (). In the multivariate analysis, household colonization was still independently associated with prolonged observed colonization time after adjusting for the other covariates. No difference was seen between the age groups 0–6 years and 7–17 years. Boys had a significantly lower median age than girls; 1 year (IQR 0-5) vs. 2 years (IQR 1–7), p = .01. In the multivariate Cox proportional hazards regression analysis, time of colonization after detection was not affected by sex but throat colonization and having household contacts were strong predictors of colonization. Children with one or more MRSA positive household contacts were older, albeit not significantly; median age 2 years (IQR 0–7) vs. 1 year (IQR 0–2), p = .06.
Table 2. Number of study participants still MRSA colonized at defined time points.
Number of positive body sites varied considerably among the children during follow-up. Once the throat was affected, it often remained colonized as a last ‘outpost’ even if findings of positive cultures at other body sites were not present. In 72 children the maximum number of positive body sites was ≥3, in 67 children 2 and in 58 children 1. The normal pattern of colonization was a successive decrease in number of affected body sites. The number of positive body sites in the last positive culture before natural clearance was 1 (n = 144), 2 (n = 35) and 3 (n = 12). In 6 cases, we did not have sufficient data to define the exact number of positive body sites before clearance. In those with one remaining positive body site, the affected site was the throat in 84 cases, the nares in 21 cases, the perineum in 14 cases (out of which 11 were younger than 3 years old and probably using diapers) and from wounds in 25 cases. All MRSA carriers without throat colonization cleared their MRSA within 48 months of carriage ().
Temporarily negative MRSA cultures
During follow-up of the 197 untreated children, 33 had one set of negative cultures and then turned positive again. Five children had two separate instances of one negative set of cultures but then again turned positive. Eighteen children had one instance with two negative sets of cultures before turning positive again and three children had two sets of negative cultures at two occasions and both times turned positive again. None of these children were declared MRSA negative until they later had three consecutive sets of negative cultures.
MRSA positive individuals at end of study
When the study period ended, nine individuals were still colonized with MRSA.
Antibiotic resistance
In the study group, bacterial resistance data were present in 281/293 (96%) individuals. Resistance was detected to clindamycin in 27% (n = 75), to fucidic acid in 32% (n = 89), to trimethoprim/sulfamethoxazole in 7% (n = 20), to rifampicin in 2% (n = 7) and to mupirocin in 1% (n = 4) of the cases.
Eradication treatment
MRSA eradication treatment was given to 96 children. Among those who received only topical eradication treatment, permanent eradication was achieved in 36% (15/42). When systemic antibiotic treatment was added 65% (35/54) acquired permanent eradication. Clindamycin (in combination with rifampicin when both were susceptible) was used in 70% (38/54) of the cases. Rifampicin resistance was seen in five of the treated cases. At treatment initiation, 79% of the children had MRSA positive throat swabs. Among them the success rate was 65% with systemic treatment and only 13% with local treatment. Sex or age did not affect the success rate. The absolute majority (96%) of the children treated with systemic antibiotics carried MRSA in the throat, whereas 57% of the individuals receiving only topical treatment carried MRSA in the throat. As only one child without throat colonization did receive systemic antibiotic treatment, no conclusions could be drawn of the efficacy of the systemic treatment on non-throat carriers. In children receiving only topical treatment, throat colonization was the only significant risk factor for continuous colonization after treatment ().
Table 3. Results of MRSA eradication treatment.
Strain constancy
Sequence of the X-region of S. aureus protein A (spa) was defined in 219/293 individuals (75%) with a total number of more than 70 different spa-types. In the untreated group, spa-type was established in 148/197 cases (75%) and the three most common spa-types were t044 (n = 22), t002 (n = 19) and t008 (n = 10). In the treated group, spa-type was established in 71/96 cases (74%) and the three most common spa-types were t008 (n = 10), t002 (n = 9) and t437 (n = 7). In 44 cases, spa-type was established more than once during follow-up of the individual, and in 8 cases a new spa-type was detected. In four of those cases, the genetic variation was too small to be conclusive regarding spa-type replacement, but in the other 4 cases the new spa-type indicated a new MRSA strain. These individuals, who had acquired a new MRSA strain, were excluded from the study. The heterogeneity of spa-types among the carriers prevented evaluation of the effect on colonization time and eradication success depending on spa-type.
PVL status
Presence of the cytotoxin PVL was evaluated in 192/293 cases (66%) and was present in 82 (43%) of them. In the untreated group PVL was present in 54/124 cases (44%) and in the treated group in 28/68 cases (41%). As expected, PVL positivity was associated with certain spa-types, which was defined in 79/82 cases. Among these PVL positive cases the most common spa-types were t044 (n = 30; 38%), t008 (n = 10; 13%) and t131 (n = 10; 13%). PVL positivity correlated with higher age (p = .041). There was an interaction with age in the multivariate analysis where presence of PVL correlated with shorter colonization time in the older age group (Hazard ratio 0.19, p = .07) but not in the younger age group. However, there were only 12 PVL positive cases in the older age group, making the result hard to interpret. Among throat carriers, PVL positivity correlated with increased eradication success in the multivariate analysis (p = .028). In the treated group there was an even distribution of spa-types among the PVL positive cases.
Discussion
We have here found that 62% of non-treated persistent MRSA colonized children, living in southern Sweden, remained colonized 1 year after detection and 28% 2 years after detection. Persistent eradication was achieved in 65% of children receiving systemic antibiotics aiming at MRSA eradication.
Throat colonization clearly resulted in a longer time of observed colonization, which could indicate that it is more difficult to clear the bacteria once the throat has been affected. This has also been demonstrated regarding S. aureus colonization in school-aged children [Citation29]. There was a great variation of number of affected body sites in most children during follow-up, which led us to abstain from performing analysis regarding relationship between number of affected body sites and observed colonization time.
Having MRSA positive household contacts also prolonged the observed colonization time. This is in coherence with previous studies [Citation13,Citation18]. Whether this is a result of a more solid colonization, due to high household bacterial burden, or to repeated reinfections is not clear.
Presence of PVL correlated with higher age in the studied children. We also found a correlation between PVL positivity and shortened colonization time in the older age group, and among the treated throat colonized individuals, PVL positivity correlated with increased eradication success. These findings have not been presented before. The PVL positive isolates were, however, distributed among a few of the most prevalent spa-types found in the material. With the small number of PVL positive cases in mind, it is hard to evaluate if differences in colonization time and treatment result can be attributed to the PVL positivity as such or to specific spa-types, which here can be a confounding factor.
The main objective of MRSA eradication treatment in low prevalent areas, where reinfections from the society is unlikely, is permanent clearance of MRSA. Benefits for the individual should be a lessened risk of endogenously derived infections and ending of precautions aiming at diminish MRSA transmission in health care, which can be considered inconvenient for the MRSA carrier. Signs of stigma affecting the mental health in MRSA carriers have been shown [Citation30].
Temporary nasal decolonisation of S. aureus during hospital stay, achieved by for example nasally applied mupirocin and chlorhexidine washes, has been shown to effectively prevent nosocomial transmission and endogenously derived infections [Citation31,Citation32], preventing septic episodes and surgical-site infections. When S. aureus is detected in the throat, local treatment has been shown to be less successful [Citation33,Citation34]. Therefore, systemic antibiotic treatment was added in most of those cases in our cohort with the achievement of 65% persistent eradication. To our knowledge, the effect of MRSA eradication treatment in children has not been studied before but our results are in line with studies in older ages [Citation13,Citation22], demonstrating 60–70% success rate in such treatments performed in all ages. Possible factors, not evaluated here, affecting the treatment effect could be local factors in the tonsils, the immune system, bacterial characteristics or bacterial burden. Studies performed in all ages have shown a higher rate of treatment failure when systemic antibiotics are used if the perineum or throat is MRSA colonized and if MRSA positive household contacts are present [Citation33,Citation35]. Reinfection from the society or household contacts is a possibility but probably rare in our material, due to the low MRSA prevalence in the society and the fact that all MRSA positive household members were treated simultaneously. Spa-typing was repeated in 44 individuals during follow-up. In only 4 cases, the spa-type was changed indicating reinfection with a new strain, supporting the theory of a low reinfection frequency outside the households.
Systemic treatment could be considered unsuitable in certain cases despite extensive colonization including the throat. If so, local treatment was sometimes used in purpose to diminish the risk of transmission to household contacts, since it has been shown that MRSA is transmitted within households [Citation16,Citation36]. In our material, topical treatment was mostly chosen in cases lacking throat colonization. Overall eradication success with topical treatment was only seen in 36%. Among throat carriers only 13% reached sustained eradication with topical treatment, which should resemble the expected natural disappearance rate.
There are several weaknesses of the design of this retrospective study, where neither follow-up routines nor treatment given were fully standardized. The observed colonization time in our study is longer than the median observed colonization time of 5.9 months (all ages) found in a study in a similar catchment area [Citation13]. In that study, however, children also carried MRSA for a longer time than the overall population (11.3 months). Individuals, given MRSA eradication treatment, were included in that data and do not represent the natural colonization time. In our study, the patients were sampled approximately every 6th month. This infrequent sampling regime makes calculated colonization time inexact and with a shorter interval between the cultures a somewhat shorter colonization time would probably have been observed. However, the different factors affecting the observed colonization time, would not have changed. We also believe that shorter, more patient inconvenient, sampling intervals could have affected compliance to a large extent, why our follow-up schedule was chosen knowingly rendering a somewhat longer follow-up time than what is biologically correct. In our study, a certain selection bias might however to some degree have shortened the observed colonization time since some individuals with infectious problems, including their MRSA positive household contacts, were selected for eradication treatment and for those no untreated observation time is available. Former studies on methicillin-susceptible S. aureus (MSSA) colonization in children [Citation8,Citation37] support the findings of a longer colonization time in children than adults. Individuals with MRSA negative samples obtained after a long lag time were excluded since it was impossible to reasonably evaluate their colonization time. Individuals, still MRSA positive with the same strain, were however included even though retransmission from relatives could not be excluded. This is however the case throughout the entire follow-up period. Having MRSA positive household contacts clearly prolongs the observed colonization time. In this study we have considered every MRSA positive child as a separate case, where we noted if positive household contacts existed or not. However, we did not have the possibility to take neither the exact number of positive household contacts nor for how long the contacts were colonized into account, which could have improved the understanding.
The young median age in our cohort, as well as the high proportion of MRSA positive household contacts, could be influenced by the MRSA screening performed in pregnant women followed by contact tracing in all children born by a MRSA positive mother. Older children were only cultured for MRSA if they were mandatory screened, contact traced or had a SSTI.
In our study population 78% of the individuals carried MRSA in the throat. A former study on long-term carriage in all ages has shown high throat carriage as well (75%) [Citation38] and, when focusing on the young, throat carriage seems to be more common [Citation39]. We primarily do not consider this finding a selection bias.
Advantages in our study are the lowered risk of reinfections after treatment due to low MRSA prevalence in the society and that MRSA positive household members were treated simultaneously. Another strength is the highly sensitive microbiological method that was used [Citation23,Citation40]. It could, however, be discussed if three consecutive negative sets of cultures during follow-up period are enough to detect all relapses. It has been suggested that a more frequent follow-up should be used for earlier relapse detection [Citation41]. In our cohort, with mainly healthy individuals, we have found our regime suitable and this is also supported by earlier evaluations [Citation13].
In conclusion, we have found that 62% of the MRSA positive children not given eradication treatment remained positive 1 year after detection. The success rate was 65% when treating MRSA colonized children with systemic antibiotics, mupirocin and a hygiene program. To confirm the eradication treatment performance, further prospective studies are needed.
Supplemental Material
Download MS Word (13.7 KB)Acknowledgements
The authors whish to thank all the participant patients and their families for their contributions. We would also like to thank the staff of the outpatient section of the Malmö division of the Infectious Disease Department, especially the registered nurses Karin Andersson, Emma Fagerstrand and Pernilla Olsson, as well as the staff of the Clinical Microbiology Laboratory in Malmö for excellent work.
Disclosure statement
The authors report no conflict of interest.
References
- Swedres/Swarm 2016 [Internet]. Consumption of antibiotics and occurrence of antibiotic resistance in Sweden. 2017. Available from: https://www.folkhalsomyndigheten.se/pagefiles/31498/Swedres-Svarm-2016-16124.pdf
- Cosgrove SE, Sakoulas G, Perencevich EN, et al. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59.
- Gould IM. Costs of hospital-acquired methicillin-resistant Staphylococcus aureus (MRSA) and its control. Int J Antimicrob Agents. 2006;28:379–384.
- Song X, Perencevich E, Campos J, et al. Clinical and economic impact of methicillin-resistant Staphylococcus aureus colonization or infection on neonates in intensive care units. Infect Control Hosp Epidemiol. 2010;31:177–182.
- den Heijer CD, van Bijnen EM, Paget WJ, et al. Prevalence and resistance of commensal Staphylococcus aureus, including methicillin-resistant S aureus, in nine European countries: a cross-sectional study. Lancet Infect Dis. 2013;13:409–415.
- Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46(Suppl 5):344–349.
- Crum NF, Lee RU, Thornton SA, et al. Fifteen-year study of the changing epidemiology of methicillin-resistant Staphylococcus aureus. Am J Med. 2006;119:943–951.
- Wertheim HF, Melles DC, Vos MC, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762.
- Kluytmans JA, Mouton JW, Ijzerman EP, et al. Nasal carriage of Staphylococcus aureus as a major risk factor for wound infections after cardiac surgery. J Infect Dis. 1995;171:216–219.
- Kalmeijer MD, van Nieuwland-Bollen E, Bogaers-Hofman D, et al. Nasal carriage of Staphylococcus aureus is a major risk factor for surgical-site infections in orthopedic surgery. Infect Control Hosp Epidemiol. 2000;21:319–323.
- Marschall J, Muhlemann K. Duration of methicillin-resistant Staphylococcus aureus carriage, according to risk factors for acquisition. Infect Control Hosp Epidemiol. 2006;27:1206–1212.
- Scanvic A, Denic L, Gaillon S, et al. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis. 2001;32:1393–1398.
- Larsson AK, Gustafsson E, Nilsson AC, et al. Duration of methicillin-resistant Staphylococcus aureus colonization after diagnosis: a four-year experience from southern Sweden. Scand J Infect Dis. 2011;43:456–462.
- Vriens MR, Blok HE, Gigengack-Baars AC, et al. Methicillin-resistant Staphylococcus aureus carriage among patients after hospital discharge. Infect Control Hosp Epidemiol. 2005;26:629–633.
- Robicsek A, Beaumont JL, Peterson LR. Duration of colonization with methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2009;48:910–913.
- Lucet JC, Paoletti X, Demontpion C, et al. Carriage of methicillin-resistant Staphylococcus aureus in home care settings: prevalence, duration, and transmission to household members. Arch Intern Med. 2009;169:1372–1378.
- Mitsuda T, Arai K, Fujita S, et al. Epidemiological analysis of strains of methicillin-resistant Staphylococcus aureus (MRSA) infection in the nursery; prognosis of MRSA carrier infants. J Hosp Infect. 1995;31:123–134.
- Cluzet VC, Gerber JS, Nachamkin I, et al. Duration of Colonization and Determinants of Earlier Clearance of Colonization With Methicillin-Resistant Staphylococcus aureus. Clin Infect Dis. 2015;60:1489–1496.
- van Rijen M, Bonten M, Wenzel R, et al. Mupirocin ointment for preventing Staphylococcus aureus infections in nasal carriers. Cochrane Database Syst Rev. 2008; Cd006216.
- Muhlebach MS, Beckett V, Popowitch E, et al. Microbiological efficacy of early MRSA treatment in cystic fibrosis in a randomised controlled trial. Thorax. 2017;72:318–326.
- Meyer V, Kerk N, Mellmann A, et al. MRSA eradication in dermatologic outpatients – theory and practice. J Dtsch Dermatol Ges. 2012;10:186–196.
- Ammerlaan HS, Kluytmans JA, Berkhout H, et al. Eradication of carriage with methicillin-resistant Staphylococcus aureus: effectiveness of a national guideline. J Antimicrob Chemother. 2011;66:2409–2417.
- Nilsson P, Alexandersson H, Ripa T. Use of broth enrichment and real-time PCR to exclude the presence of methicillin-resistant Staphylococcus aureus in clinical samples: a sensitive screening approach. Clin Microbiol Infect. 2005;11:1027–1034.
- Nilsson AC, Janson H, Wold H, et al. LTX-109 is a novel agent for nasal decolonization of methicillin-resistant and -sensitive Staphylococcus aureus. Antimicrob Agents Chemother. 2015;59:145–151.
- Reischl U, Linde HJ, Metz M, et al. Rapid identification of methicillin-resistant Staphylococcus aureus and simultaneous species confirmation using real-time fluorescence PCR. J Clin Microbiol. 2000;38:2429–2433.
- Murakami K, Minamide W, Wada K, et al. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 1991;29:2240–2244.
- Brakstad OG, Aasbakk K, Maeland JA. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J Clin Microbiol 1992;30:1654–1660.
- Petersson AC, Olsson-Liljequist B, Miorner H, et al. Evaluating the usefulness of spa typing, in comparison with pulsed-field gel electrophoresis, for epidemiological typing of methicillin-resistant Staphylococcus aureus in a low prevalence region in Sweden 2000–2004. Clin Microbiol Infect. 2010;16:456–462.
- Williamson DA, Ritchie S, Keren B, et al. Persistence, discordance and diversity in Staphylococcus aureus nasal and oropharyngeal colonization in school-aged children. Pediatr Infect Dis J. 2016;35:744–748.
- Rump B, De Boer M, Reis R, et al. Signs of stigma and poor mental health among carriers of MRSA. J Hosp Infect. 2017;95:268–274.
- Sai N, Laurent C, Strale H, et al. Efficacy of the decolonization of methicillin-resistant Staphylococcus aureus carriers in clinical practice. Antimicrob Resist Infect Control. 2015;4:56.
- Bode LG, Kluytmans JA, Wertheim HF, et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N Engl J Med. 2010;362:9–17.
- Mollema FP, Severin JA, Nouwen JL, et al. Successful treatment for carriage of methicillin-resistant Staphylococcus aureus and importance of follow-up. Antimicrob Agents Chemother. 2010;54:4020–4025.
- Harbarth S, Dharan S, Liassine N, et al. Randomized, placebo-controlled, double-blind trial to evaluate the efficacy of mupirocin for eradicating carriage of methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:1412–1416.
- Ammerlaan HS, Kluytmans JA, Berkhout H, et al. Eradication of carriage with methicillin-resistant Staphylococcus aureus: determinants of treatment failure. J Antimicrob Chemother. 2011;66:2418–2424.
- Johansson PJ, Gustafsson EB, Ringberg H. High prevalence of MRSA in household contacts. Scand J Infect Dis. 2007;39:764–768.
- Armstrong-Esther CA. Carriage patterns of Staphylococcus aureus in a healthy non-hospital population of adults and children. Ann Hum Biol. 1976;3:221–227.
- Bocher S, Skov RL, Knudsen MA, et al. The search and destroy strategy prevents spread and long-term carriage of methicillin-resistant Staphylococcus aureus: results from the follow-up screening of a large ST22 (E-MRSA 15) outbreak in Denmark. Clin Micrbiol Infect. 2010;16:1427–1434.
- Mertz D, Frei R, Periat N, et al. Exclusive Staphylococcus aureus throat carriage: at-risk populations. Arch Intern Med. 2009;169:172–178.
- Bocher S, Middendorf B, Westh H, et al. Semi-selective broth improves screening for methicillin-resistant Staphylococcus aureus. J Antimicrob Chemother. 2010;65:717–720.
- Lekkerkerk WS, Uljee M, Prkic A, et al. Follow-up cultures for MRSA after eradication therapy: are three culture-sets enough? J Infect. 2015;70:491–498.
