Abstract
Background: Pseudomonas aeruginosa isolates from Cystic fibrosis (CF) patients are growing slowly, and frequently rendering automated susceptibility testing unsuitable. Colistin is an important antibiotic for treatment of P. aeruginosa infections. Broth microdilution is the only EUCAST endorsed antimicrobial susceptibility test for colistin. The VIZION™ device aids in reading broth microdilution plates and allows safe data transfer to laboratory information systems. In this study, reproducibility between visual MIC readout and readout, employing the VIZION™ device was assessed in susceptibility testing of colistin and beta-lactam antibiotics in P. aeruginosa isolates from CF patients.
Methods: Fifty-six unique P. aeruginosa isolates were derived from respiratory secretions of CF patients. Susceptibility testing was performed using commercially available microdilution plates. MIC readout by VIZION™ was compared to visual readout aided by a mirror (reference test).
Results: Pseudomonas aeruginosa isolates displayed significantly slower growth rates compared to quality control isolates. Colistin exact MIC agreement between VIZION™ and visual readout after 24 and 48 h incubation, respectively, was 82% and 95%, essential MIC agreement was 98% and 100%, categorical agreement was 98% and 98% and reliability (weighted kappa) was 0.95 (95% CI = 0.91–0.99) and 0.99 (95% CI = 0.97–1.00). For all five antibiotics, the total number of errors (using VIZION™, in comparison with visual readout) decreased from 15 (5%) to 10 (4%) after 24 and 48 h incubation, respectively.
Conclusions: VIZION™ readout reproducibly determines MIC values in comparison with visual readout after 24 h of incubation. Reproducibility between the VIZION™ and visual readout increases after prolonged incubation of 48 h.
Introduction
Colistin is considered a last resort antibiotic for multi-drug resistant Gram-negative bacteria such as Pseudomonas aeruginosa, but isolates from CF patients are often slowly growing and reading of broth dilution MIC values, may be challenging. At present many European clinical microbiology laboratories will need to introduce broth dilution colistin susceptibility testing [Citation1,Citation2]. Disk diffusion is unreliable because the colistin molecule poorly diffuses into agar [Citation3]. Likewise, gradient strip tests underestimate Minimal Inhibitory Concentration (MIC) values leading to unacceptably high major error rates [Citation4,Citation5]. A high false susceptibility rate was reported for colistin using the Phoenix (BD) system in Enterobacteriaceae isolates [Citation6], and the manufacturer confirmed this as a warning on 15 January 2018. As of 19 June 2017, BioMérieux warned European Vitek users for too many major errors in automated colistin susceptibility testing with multiple different Vitek Gram-negative cards. Moreover, the European Committee on Antimicrobial Susceptibility Testing (EUCAST) and the Clinical and Laboratory Standards Institute (CLSI) published a joint statement warning that all colistin susceptibility testing requires inclusion of quality-control strains and that until further notice only broth microdilution (BMD) methodology is regarded as acceptably precise [Citation7].
Compared with BMD according to CLSI and EUCAST (ISO20776-1) standards, commercial BMD with standard freeze-dried antibiotic panels is less laborious and more readily available for the routine clinical microbiology laboratory. Moreover, computer aided readout systems, which facilitate data storage and communication to laboratory information systems, possibly reduce the risk of human error in the reporting of antimicrobial test result. The VIZION™ device used in combination with SWIN software (Thermo Fisher, Waltham, MA, USA) can be used to collect and store visual readings of broth microdilution plates and, if connected to the laboratory information system, communicates MIC data, possibly reducing the risk of human error in data transfer. Nevertheless, in comparison with automated susceptibility tests, VIZION™ reading requires more extensive hands-on time and MIC reading skills. The objective of the current study is to determine reproducibility between visual MIC reading, aided by use of a mirror (‘visual readout’), and MIC reading using the VIZION™ device (‘VIZION™ readout’) of a commercial BMD method (Sensititre™, Thermo Fisher) for antibiotic susceptibility testing of colistin and four beta-lactam antibiotics in P. aeruginosa isolates from patients with CF.
Methods
Sixty-one stored P. aeruginosa isolates, obtained from respiratory secretions of CF patients collected at the University Medical Center Utrecht, the Netherlands (one isolate per patient), were thawed. After thawing, identity of P. aeruginosa was reconfirmed for all isolates (for details, see Supplementary information). Isolates with no growth in positive control wells of the broth dilution assay after 24 h incubation were excluded (n = 5), because the 24 h reading results are invalid and cannot be compared to the 48 h reading results. In 2/5 isolates, 48 h prolonged incubation did not result in readable results. In 3/5 isolates only faint growth was observed in the positive control wells after 48 h. All isolates (n = 56) were phenotypically characterized when grown on Luria-Bertani agar and growth rate was assessed using a Bioscreen C™ device (Turku, Finland) [Citation8,Citation9].
Antibiotic susceptibility testing was performed using freeze-dried 96-well plates (EURGNCOL, Sensititre™, Thermo Fisher) according to the manufacturer’s protocol. Inoculations were aided by AIM™ (Thermo Fisher) inoculation device. The following antibiotic concentration ranges are included in the EURGNCOL plate: colistin (0.25–8 mg/L), piperacillin-tazobactam (1–32 mg/L, tazobactam 4 mg/L), ceftolozane-tazobactam (0.25–8 mg/L, tazobactam 4 mg/L), ceftazidime-avibactam (1–16 mg/L, avibactam 4 mg/L) and meropenem (0.12–16 mg/L). Results were read visually using a mirror (‘visual readout’) and aided by the VIZION™ device (‘VIZION readout’). Skipped wells (as defined by one well of no growth followed by at least one well with growth in a one 2-fold dilution lower concentration of the same antibiotic) were ignored. The MIC was defined as the lowest concentration of an antimicrobial agent that completely inhibited the growth. Regrowth was considered as contamination and ignored. Two quality-control strains (P. aeruginosa ATCC27853 and Escherichia coli NCTC13846) were included and all isolates were checked for purity. Klebsiella pneumoniae ATCC700603, required for quality-control of the inhibitory component of β-lactam–β-lactamase inhibitor combination antibiotic drugs, was not included due to a limited availability of freeze-dried antibiotic panels. A flowchart of the study design is displayed in Supplemental Figure 1.
Figure 1. Growth curves of Pseudomonas aeruginosa clinical CF isolates (n = 56, gray lines) and quality control isolates (black lines).
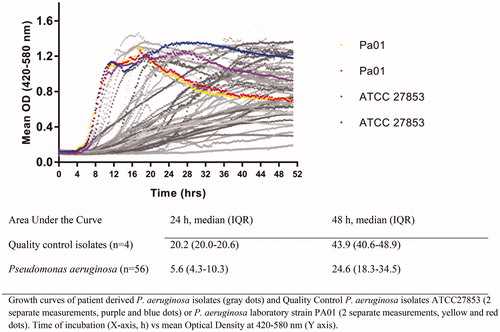
Reliability, defined as the ability to effectively distinguish low from high MIC-values, and agreement, defined as the degree to which MIC-values are identical, were estimated between VIZION™ and visual readout after 24 and 48 h of incubation. Reliability was expressed in weighted kappa taking into account the possibility of agreement occurring by chance. Agreement between VIZION™ readout and visual readout was quantified after 24 and 48 h incubation. Agreement was expressed as exact and essential (within one two-fold dilution) MIC agreement and categorical agreement, with categories interpreted according to EUCAST 2017 interpretative criteria for P. aeruginosa. Differences in area under the growth curves were tested using the Wilcoxon rank sum test.
Results
Pseudomonas aeruginosa isolates (n = 56) were phenotypically heterogeneous and displayed slower growth rates in comparison with quality-control isolates (, Supplemental Table 1 and Supplemental Figure 2). The median area under the growth curves of the quality control isolates’ differed significantly (p < .05) from the median area under the growth curves of the patient isolates’ with a ratio of 3.6 and 1.8 after 24 and 48 h incubation, respectively.
Table 1. Visual and VIZION™ antibiotic susceptibility MIC and SIR category results after 24 h of incubation (n = 56).
MIC values of the two QC strains (P. aeruginosa ATCC27853 and E coli NCTC13846) were on target or within target range in 10 repeated measurements for all antibiotics tested (Supplemental Table 2). Purity check resulted in pure cultures in all isolates. Of all 56 analyzed isolates, 22 (39%) were piperacillin-tazobactam resistant, 11 (20%) were meropenem-resistant, 8 (14%) were colistin-resistant and 19 (34%) were resistant to more than one of the tested antibiotics as measured with visual readout after 24 h incubation ().
Table 2. Reliability and agreement between VIZION and visual readout of Sensititre EURGNCOL broth dilution of P. aeruginosa isolates from CF patients (n = 56).
After 24 h incubation, colistin exact MIC agreement between VIZION™ readout and visual readout was 82%, with 98% essential MIC agreement, 98% categorical agreement and a reliability (weighted kappa) of 0.95 (95% CI = 0.91–0.99) as shown in and Supplemental Table 3. In total, for all 5 tested antibiotics, 3 very major (VIZION™ S visual R), 5 major (VIZION™ R visual S) and 7 minor errors (meropenem only) occurred. The median area under the growth curves of the isolates’ at 24 h incubation was 4 (IQR 3–10) versus 6 (IQR 5–9), in 15 isolates with errors recorded versus 41 clinical isolates without recorded errors, respectively (p = .69).
After 48 h incubation, colistin exact MIC agreement between VIZION™ readout and visual readout increased to 95%, with an essential MIC agreement of 100%, a categorical agreement of 98% and a weighted kappa of 0.99 (95% CI = 0.97–1.00). For colistin, MIC cross tabulation tables between VIZION™ and visual MIC readout after 24 and 48 h incubation are shown in Supplemental Table 3. Regarding all tested antibiotics, the number of isolates categorized as susceptible decreased, and the number of isolates scored as resistant increased. The total number of errors of VIZION™ readout in comparison with visual readout decreased from 15 (5%) to 10 (4%).
Discussion
This study demonstrates that MIC readout using the VIZION™ device of a commercial BMD method reproducibly determines MIC values in comparison with visual readout after 24 h incubation. The VIZION™ device may aid traceability of BMD susceptibility test results. Nevertheless, VIZION™ readout is not exactly the same as visual readout as it is mediated by illumination, a camera, software and a computer screen. Even for a rather difficult bug-drug combination, such as colistin in a heterogeneous population of P. aeruginosa isolates from CF patients, we found VIZION™ to be reproducible in comparison with visual reading of BMD results. Moreover, reproducibility between VIZION™ and visual readout increased after 48 h incubation. Most isolates were slowly growing and therefore prolonged incubation for P. aeruginosa from CF patients seems advisable in case of faint growth in the control well.
At present, EUCAST endorses broth microdilution according to ISO20776-1 for susceptibility testing of colistin only, whereas commercial BMD may be a reasonable alternative. Recently five commercial BMD and two gradient test methods in E. coli, K. pneumoniae, P. aeruginosa and Acinetobacter spp isolates have been compared with BMD according to the ISO20776-1 standard [Citation10]. In this study, 21 P. aeruginosa isolates were included, of which nine (43%) were colistin resistant defined by an MIC >2 mg/L. The study did not evaluate exact agreement but commercial BMD generally performed well with best correlations observed for Sensititre and two Micronaut tests (essential agreements ranging between 91–100% for all species investigated). For Sensititre one major error and no very major error occurred. In contrast performance of the two gradient strip tests was unacceptable. A second study comparing Sensititre™ commercial BMD with BMD according to modified CLSI criteria, observed a colistin 62% essential and 96% categorical agreement with no false susceptibility results in 107 Gram-negative bacilli including 60 P. aeruginosa isolates after 16–20 h incubation [Citation4]. However, in this study polysorbate-80, a surfactant which mitigates colistin adsorption to the polystyrene microplates, was added. EUCAST advises against the use of additives in BMD testing as the effect of polysorbate-80 on bacterial viability has not been well studied. A third study, without addition of polysorbate-80, demonstrated high sensitivity of Sensititre™ colistin susceptibility testing for mcr-1 positive Enterobacteriaceae [Citation11]. Although a high concordance of Sensititre™ colistin susceptibility testing to BMD was found, CLSI performance standards (very major error rate ≤1.5% and ME rate of ≤3%) were not met. Possibly, too few resistant isolates were investigated with the reference standard (broth microdilution) leading to high error rates. Second, of all ME found resistant in Sensititre™ colistin susceptibility testing but susceptible in BMD, most occurred in mcr-1 positive isolates. Therefore, it might be argued that Sensititre™ colistin susceptibility testing outperforms the gold standard in this respect.
Advantages of commercial BMD testing in P. aeruginosa isolates from patients with CF are several. First, it is less complex and laborious in comparison with BMD according to ISO20776-1 and may thereby lead to reduced error rates. Second, VIZION™ readout may aid to determine MIC values and improve traceability of test results with automated transfer of results into the laboratory information system. Disadvantages include that, for slow-growing P. aeruginosa isolates, prolonged incubation may be required to optimize readout and that clinical data relating to such readouts after prolonged incubation are lacking. Finally, the reproducibility of VIZION™ readout in comparison with visual readout found in this work may not be generalizable to other slowly growing bacteria, e.g. mycobacteria.
Strength of the present study includes the well-phenotyped set of P. aeruginosa isolates obtained from CF patients. However, for colistin inhalation treatment, often administered in CF patients, a broader range of colistin MIC results, above 8 mg/L in the here tested EURGNCOL plate, might be interesting for research purposes. In this study 8 out of 56 isolates (14%) were classified as colistin resistant (MIC >2 mg/L) which limits power for the detection of very major errors. Lastly, we do not have definitive proof that the isolates represent strict independent isolates. Care was taken to use only one isolate per patient. Nevertheless, ‘epidemic clones’, adapted to the micro-environment of bronchi from CF patients, are prevalent amongst CF patients from our institution [Citation12].
In conclusion, VIZION™ readout of a commercial BMD method (Sensititre, Thermo Fisher) reproducibly determines MIC values in comparison with visual readout after 24 h incubation. Forty-eight hours prolonged incubation of these slowly growing isolates increases reproducibility of the VIZION™ readout system in comparison with visual reading.
Supplemental Material
Download MS Word (782.8 KB)Acknowledgements
We are grateful to Kok van Kessel, Judith Vlooswijk and Peter van Bodegom for their help and contributions to this work. EURGNCOL plates and a temporary loan of AIM and VIZION™ devices (including SWIN software) were provided, free of charge, for the purpose of this study, by Thermo Fisher.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev. 2017;30:557–596.
- Rutter WC, Burgess DR, Burgess DS. Increasing incidence of multidrug resistance among cystic fibrosis respiratory bacterial isolates. Microb Drug Resist. 2017;23:51–55.
- Tan TY, Ng LS. Comparison of three standardized disc susceptibility testing methods for colistin. J Antimicrob Chemother. 2006;58:864–867.
- Hindler JA, Humphries RM. Colistin MIC variability by method for contemporary clinical isolates of multidrug-resistant Gram-negative bacilli. J Clin Microbiol. 2013;51:1678–1684.
- Rojas LJ, Salim M, Cober E, et al. Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: laboratory detection and impact on mortality. Clin Infect Dis. 2017;64:711–718.
- Jayol A, Nordmann P, Lehours P, et al. Comparison of methods for detection of plasmid-mediated and chromosomally encoded colistin resistance in Enterobacteriaceae. Clin Microbiol Infect. 2018;24:175–179.
- European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrobial susceptibility testing of colistin – problems detected with several commercially available products. 2018 [updated February 2018]. Available from: http://www.eucast.org/ast_of_bacteria/warnings/
- Cullen L, Weiser R, Olszak T, et al. Phenotypic characterization of an international Pseudomonas aeruginosa reference panel: strains of cystic fibrosis (CF) origin show less in vivo virulence than non-CF strains. Microbiology. 2015;161:1961–1977.
- Mayer-Hamblett N, Rosenfeld M, Gibson RL, et al. Pseudomonas aeruginosa in vitro phenotypes distinguish cystic fibrosis infection stages and outcomes. Am J Respir Crit Care Med. 2014;190:289–297.
- Matuschek E, Ahman J, Webster C, et al. Antimicrobial susceptibility testing of colistin - evaluation of seven commercial MIC products against standard broth microdilution for Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, and Acinetobacter spp. Clin Microbiol Infect. 2018;24:865–870.
- Chew KL, La MV, Lin RTP, et al. Colistin and polymyxin B susceptibility testing for carbapenem-resistant and mcr-positive Enterobacteriaceae: comparison of sensititre, microScan, vitek 2, and eEtest with broth microdilution. J Clin Microbiol. 2017;55:2609–2616.
- van Mansfeld R, Willems R, Brimicombe R, et al. Pseudomonas aeruginosa genotype prevalence in Dutch cystic fibrosis patients and age dependency of colonization by various P. aeruginosa sequence types. J Clin Microbiol. 2009;47:4096–4101.
