Abstract
Background: Along with the current development of molecular diagnostic methods of respiratory viruses, the bedside patient sampling techniques need to be evaluated. We here asked the question whether the addition of an oropharynx swab to the traditional nasopharynx swab might improve the diagnostic yield of multiplex PCR analysis. Ct values from the two sampling sites were compared as well as patient tolerability.
Methods: In an emergency department in Malmö, Sweden, 98 adult patients with respiratory disease were sampled both from the nasopharynx and oropharynx for virus diagnostics by PCR.
Results: Influenza (AH1, AH3, B), human metapneumovirus (hMPV) or respiratory syncytial virus (RSV) were detected by PCR in 58 subjects. The diagnostic yield was improved by combining nasopharyngeal and oropharyngeal sampling – a virus was detected in another 6 patients compared to traditional nasopharyngeal sampling (p = .031, McNemar’s test). In 38/55 subjects viral load was higher in the nasopharynx than in the oropharynx. Self-reported discomfort was significantly lower from oropharyngeal sampling than from nasopharyngeal sampling.
Conclusions: Adding an oropharynx sample to a nasopharynx sample increased the diagnostic yield of respiratory viruses. Oropharyngeal sampling was well tolerated.
Introduction
Patients with respiratory tract infections are common in the emergency department, particularly in the wintertime and especially during the influenza seasons. The importance of determining the causative agent is undisputable, as there are a number of clinically significant microorganisms – virus and bacteria – requiring different pharmacological treatment and with different risk for in-hospital transmission [Citation1–5]. The detection of viral agents in the respiratory tract for which specific treatment is not yet available, has been shown to diminish the use of antibiotics [Citation6,Citation7]. Diagnosis of respiratory tract infection can also at times halt further investigations of the patient.
The laboratory means of diagnosing influenza and other viral respiratory pathogens have improved lately with the implementation of sensitive and rapid PCR methods [Citation4]. However, the bed side sampling techniques have not equally been evaluated in relation to the new diagnostic methods and it is yet to be shown which sampling technique is the most optimal regarding sensitivity, specificity and patient discomfort. In clinical practise the gold standard technique when sampling upper respiratory tract infections has been nasopharyngeal aspirates. With access to more sensitive laboratory techniques, less invasive sampling methods, such as flocked nasal swabs, has become a widely used alternative [Citation8,Citation9].
Recent studies indicate that sampling from the oropharynx might be an alternative although probably not as efficient as the nasopharynx sample and optimal sampling site might differ between pathogens [Citation10,Citation11].
We investigated whether adding an oropharyngeal swab to the traditional nasopharyngeal swab when sampling for respiratory agents to be analysed by multiplex PCR might improve the diagnostic yield. Sample site detection rates and diagnostic coherence between nasopharynx and oropharynx were evaluated. Cycle threshold (Ct) values, a semi-quantitative measure of viral load, shown to vary with duration and severity of disease, were compared in the consecutive samples, to assess differences in tentative viral load [Citation12,Citation13]. Sampling discomfort reported by patients was evaluated.
Material and methods
Setting
The study was conducted at the emergency department of Skåne University Hospital, Malmö, in Southern Sweden. This tertiary care hospital had 640 beds and 85,000 emergency department visits yearly when the study was conducted.
Study design
Patients with acute onset of cough, sore throat, dyspnoea, and/or rhinitis, aged ≥18 years seeking care at Malmö emergency department were randomly selected for inclusion in the study by the ordinary staff. No exclusion criteria were applied. Only study participants with samples obtained from both nasopharynx and oropharynx were included in the analyses. The study was conducted January to March 2014 and February to April 2015, covering two influenza seasons.
After informed consent was obtained, consecutive samples from the nasopharynx and the oropharynx were harvested. The patients were asked to rate the sampling discomfort experienced in the respective procedure. An arbitrary rating scale (1–4) was used with (1) representing no discomfort, and (4) intolerable discomfort. The duration of disease and type of symptoms were noted. The patients were thereafter treated as per regular practise in the emergency department. The results from pulmonary X-ray performed in the clinical practice and the highest recorded value for each of C-reactive protein (CRP) and white blood cell count (WBC) for each patient were noted.
The study was approved by the Lund University regional ethics committee, file number 2013/613.
Sample collection
The respiratory tract samples were obtained by any of the regular nurses of the emergency department. The yearly update in the beginning of each influenza season for the nursing staff of the emergency department on how to perform nasopharyngeal sampling was extended during the seasons when the study was performed, by adding information on how to perform oropharyngeal sampling. The nasopharyngeal sample was obtained by a nurse inserting a flocked nylon swab into the nasopharynx and rotating it twice. The same nurse thereafter inserted a new swab into the oropharynx and stroked it twice on the back oropharyngeal wall. Nasopharyngeal, Copan Minitip FloqSwabs with molded break point (Copan Flock Technologies Srl, Brescia, Italy) were used for both samples. After sampling the swabs were each put in a separate sterile test tube containing 1 mL of sterile saline solution.
Virus detection
Both the nasopharyngeal and the oropharyngeal samples were frozen at −20 °C at the Department of Clinical Microbiology, Lund for later analysis with PCR for study purpose.
All samples were analysed for the presence of respiratory viruses by real-time PCR at the Department of Microbiology, Lund, a diagnostic laboratory accredited according to ISO 18189 (Swedac). The analyses included influenza A and B, human metapneumovirus (hMPV) and respiratory syncytial virus (RSV). A slightly modified version of the multiplex real-time PCR-assay adopted by Østby et al. [Citation5] including subtyping of influenza A, was used. MagNA Pure 96 (Roche Diagnostics) was used for the extraction of RNA from 200 µL of the sample without any other pre-treatment performed. The amplifications were performed on an ABI 7500 real-time PCR system (Applied Biosystems). The results were blinded to the laboratory technician.
Specific limits of detection for the PCR tests used in the study were not calculated when the tests were implemented. However, testing of quality assurance panels from national and international institutes (Equalis and QCMD) were tested with excellent results and have remained so during the following years. Several negative controls were included in the PCR setups. No false positive results were observed during the study. Comparison of Ct values was performed to examine the difference in tentative viral load. Low values represent a high viral load. Ct values ≥40 were classified as negative. Only differences in Ct values of >3 cycles were considered.
Gold standard: A patient was considered to be positive for a specific viral agent if the virus was detected in any of the two test sites.
Statistical methods
Viral detection rates in nasopharynx and oropharynx samples were calculated by dividing the number of positive findings of each virus, and all viruses combined, per sampling site by the total number of patients positive for that virus according to gold standard. The results from the two sampling sites were compared by McNemar’s test, for each virus and for all viruses combined. To evaluate whether oropharyngeal sampling added diagnostic value to nasopharyngeal sampling, we compared detection rates by McNemar’s test for all analysed viruses in nasopharynx only with detection when combining nasopharynx and oropharynx sampling.
Self-rated sampling discomfort on a 4-grade scale was recoded into a dichotomous variable (no/mild discomfort vs. considerable/intolerable discomfort). Discomfort from nasopharynx vs. oropharynx sampling was compared by McNemar’s test. p-Values < .05 were considered statistically significant.
Data preparation and statistical analyses were performed in SPSS 22.0.
Results
Sampling site detection rates and coherence
Ninety-eight patients (58% female, median age 55.5 years [range 22–97]) were included in the study (). Respiratory viruses were detected by PCR in 58 patients. Viruses detected were; influenza AH1 (n = 19), influenza AH3 (n = 13), influenza B (n = 13), hMPV (n = 7) and RSV (n = 6) ().
Table 1. Characteristics and clinical presentation in patients with PCR verified respiratory viruses.
Nasopharynx and oropharynx results were coherent in 42 patients (72%) () in whom the same virus was detected in both sampling sites. A virus was detected in the nasopharynx but not in the oropharynx in 10 patients; AH1 (n = 2), AH3 (n = 5), B (n = 3) and the other way around, in the oropharynx but not in the nasopharynx, in another 6 patients; A H1 (n = 3), B (n = 1) and hMPV (n = 2).
Table 2. Diagnostic matching between nasopharyngeal (NP) and oropharyngeal (OP) sampling for viral agents, detected by PCR analysis. Total N = 98. Presented as absolute numbers.
Detection rates in the nasopharynx swabs ranged from 71% (hMPV) to 100% (influenza AH3 and RSV). Oropharynx detection rates ranged from 62% (influenza AH3) to 100% (hMPV and RSV) (). No statistically significant difference in viral detection rate was found between the nasopharynx swabs and oropharynx swabs. However, by adding an oropharyngeal sample to the nasopharyngeal sample, the increase in viral detection was statistically significant (p = .031 by McNemar’s test).
Table 3. Sensitivity of NP (nasopharynx) swab and OP (oropharynx) swab. Total N = 98. p-Value compares viral detection rate and is calculated by McNemar’s test.
Clinical characteristics and treatment
Samples were obtained 1–15 days after self-reported symptom debut which is presented in along with laboratory and X-ray data of the 45 patients who had a pulmonary X-ray performed (). None of the patients had received previous antiviral treatment. Forty-three patients received inpatient care, and among those virus was detected in 20; any influenza virus (n = 11), hMPV (n = 5) and RSV (n = 4). There was one death during hospital stay, in a patient without viral detection. Empiric antiviral treatment with oseltamivir was initiated after sampling in 3 patients in total. In one of them influenza was not detected and consequently oseltamivir was discontinued. Empiric antibiotic treatment was prescribed to 38 patients (of whom 31 received inpatient care). In 14 of these a viral agent was detected.
In another eight patients admitted for inpatient care oseltamivir was prescribed after detection of influenza virus. Among 34 patients with laboratory confirmed influenza, who were discharged to home from the emergency department, oseltamivir was prescribed in three patients.
Viral load in the nasopharynx and the oropharynx
Of the 58 patients with a positive virus finding, Ct values were available for 55. In most patients (n = 38) the Ct values were considerably lower (median 10 cycles, range 3–18) in the nasopharyngeal swabs compared to the corresponding oropharyngeal swabs indicating a higher viral load in the nasopharynx than in the oropharynx (influenza AH1 n = 11, influenza AH3 n = 12, influenza B n = 7, RSV n = 4, hMPV n = 4). In 10 patients there was no significant difference (< 3 cycles) in Ct values between the two samples (influenza AH1 n = 5, influenza B n = 4, hMPV n = 1) and in 7 patients the Ct values were lowest (median 6 cycles, range 4–18) in the oropharyngeal sample (influenza AH1 n = 3, influenza AH3 n = 1, influenza B n = 1, RSV n = 1, hMPV n = 1). A low viral load was only seen in both samples in two cases. Among the 10 samples only positive in the nasopharyngeal swab, the viral load was considered high (Ct value <30) in six samples and low (Ct value ≥35) in four samples. Among the six samples only positive in the oropharyngeal swab the viral load was considered high in two samples, medium high (Ct value 30–35) in one sample and low in three samples. These samples were taken 1–5 days after disease onset. In one patient with influenza AH1 pneumonia was diagnosed.
Patient discomfort
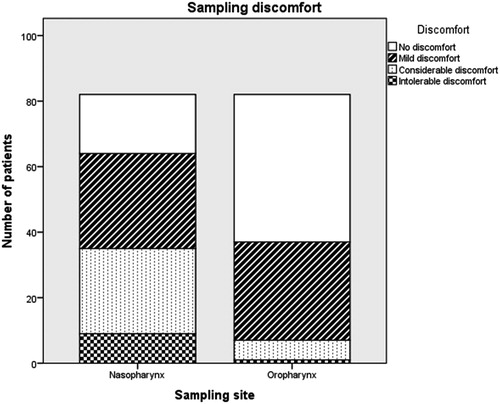
Eighty-two individuals rated the sampling discomfort from both sampling sites. Self-rated sampling discomfort on a 4-grade scale was recorded as a dichotomous variable (no/mild discomfort vs. considerable/intolerable discomfort). Reported discomfort was significantly lower from oropharyngeal sampling than from nasopharyngeal sampling (p < .001, calculated by McNemar’s test). Forty-seven subjects (57%) reported no or mild discomfort from nasopharyngeal sampling, and 75 individuals (91%) rated the discomfort from the oropharyngeal sampling as non-existent or mild ().
Discussion
We found that adding an oropharynx sample to routinely performed nasopharyngeal sampling significantly increased the diagnostic yield of respiratory viruses (influenza A or B, hMPV or RSV) among adult patients in a real-life emergency department setting. In the majority of patients, the virus could be detected in samples both from the nasopharynx and the oropharynx, and we did not find support for choosing one sampling site over the other.
To our knowledge, the diagnostic efficacy of a combination of the two sample types have not previously been evaluated when sampling is performed under ordinary emergency department conditions.
However, studies from other settings than an emergency department have shown different results regarding detection rates in nasopharynx and oropharynx samples. In one study of otherwise healthy health care personnel with 53 positive cases of influenza A (swab type not specified), detection rates were the same (87%) in nasopharynx and oropharynx samples [Citation14]. In a study of 32 elderly patients with confirmed influenza, the sensitivity was significantly lower for influenza A and B in oropharynx (63%) compared to nasopharynx (78%) when retested by flocked swabs within three days of admittance to hospital. [Citation11].
In another study of 80 inpatients with influenza A or B positive lower respiratory tract infection, cotton tipped nasopharyngeal samples were compared with oropharyngeal samples and nasopharyngeal washes. The sampling sensitivity was 97% for nasopharyngeal washes, 76% for nasopharyngeal and 56% for oropharyngeal sampling [Citation15]. If flocked nylon swabs had been used the swabbing might have rendered a higher overall detection rate since they have been shown to yield a somewhat higher sensitivity than rayon tipped swabs [Citation16,Citation17].
Respiratory sampling methods have been more extensively evaluated in children, although to our knowledge emergency department studies have not been performed in the younger age group. In a respiratory surveillance site study performed mainly in children age <5 years, any virus was detected in 1402 participants by one of paired nasopharynx and oropharynx swabs. Influenza A (H1N1) detection rates were 74% in the nasopharynx and 90% in the oropharynx. Influenza B detection rates were 83% in nasopharynx and 61% in oropharynx samples [Citation18].
The differing results in these prevalence studies performed in subjects with different ages indicate that the sensitivity of respiratory sampling methods found in children could not automatically be extrapolated to adults. Age specific studies are necessary.
We noted lower Ct values for tests on nasopharyngeal samples than on oropharyngeal samples in general in the influenza patients, indicating a higher viral presence in the nasopharynx, consistent with the result in a recent Norwegian study [Citation11]. Even though no statistically significant difference between viral detection rate in nasopharyngeal and oropharyngeal sampling was noted in this study, the difference in Ct values indicates higher probability of detecting influenza virus in the nasopharynx. The reason for retrieving negative samples or achieving high Ct values could be low viral load in the sampled site, however, bed side sampling errors cannot be ruled out.
We also explored the sampling related discomfort experienced by the patient in nasopharynx and oropharynx sampling; a comparison which has formerly not been extensively described. We found that 91% of the subjects perceived no or slight discomfort when undergoing the oropharyngeal swabbing compared to 57% of the subjects undergoing nasopharyngeal swabbing. Formerly in an emergency department patient study, sampling under ordinary clinical circumstances in the acute phase of a respiratory tract infection no or slight discomfort was perceived from flocked nasal sampling by 23/35 (67%) patients. Unbearable discomfort was reported by 2 patients [Citation8]. In our study nasopharyngeal sampling was found intolerable by 9/82 patients and the oropharyngeal sampling by one patient. It cannot be ruled out that the sampling order in our study have affected the self-reported perceived discomfort. The oropharynx was always swabbed after the nasopharynx and some patients might have underestimated the discomfort from the oropharynx sample due to a feeling of relief compared to the nasopharynx sampling. Others might have overestimated the oropharynx discomfort due to a lassitude of the entire sampling procedure. This is not a matter which we investigated, but the total effect from the consistent sampling order on the perceived discomfort reported should be low.
In another study discomfort of nasal wash, nasal brush, nasopharyngeal aspirate and nasal swabbing (cotton tipped swab) was compared in 39 adult patients with a common cold. The nasal swabbing was rated with the lowest rate of discomfort of these sampling techniques with an average of 2.54 on a 5 grade scale [Citation19]. Two other studies focusing on infants showed nasal swabs to be less discomforting than nasopharyngeal aspirates and nasal washes [Citation20,Citation21]. Studies exploring sampling discomfort of oropharyngeal sampling could not be found.
We have interestingly found that only 2/12 patients with influenza needing hospital care during an influenza epidemic were prescribed empiric influenza treatment. However, 31 of the 43 hospitalized patients with symptoms of respiratory tract infections were prescribed empiric antibiotic treatment. We believe that fast and accurate tools for the detection of infective agents could result in improved and earlier correct treatment of patients with airway infections. To minimize the cost of dual samples, pooling of the samples when carrying out the analysis has been suggested and shown not to compromise the results [Citation22].
Influenza H1N1 has previously been observed to have a proportionally higher detection rate in bronchoscopic lavage in comparison to H3N2 - explained by viral attachment further down in the airways [Citation23]. In coherence with this, a better detection rate in the oropharynx for H1N1 could be suspected as our data suggests, although the number of included patients is too low for a valid conclusion, which is a limit to this study.
This study has other limitations that need to be taken into consideration when interpreting the results. The random selection of patients for inclusion might have led to a selection bias towards inclusion of less severely ill patients, as they would be better suited to understand the study information and to sign the consent form. Thus this study cannot verify that the results are applicable to the most severely ill emergency department patients. However, we would hypothesize that the most severely ill patients could have a higher likelihood of a lower respiratory tract infection, thus indicating a greater value of adding an oropharynx sample.
For simplicity, we used nasopharyngeal swabs for both sampling sites, which may have affected the detection rate in the oropharynx. Use of a sturdier oropharyngeal swab for the throat sample might have rendered a higher detection rate. In a stressful emergency department setting, we considered the risk of mixing up swabs, especially in severe cases. For the sake of minimizing the risk of errors we accepted a slightly lower chance of viral detection in the oropharynx.
Another limitation of the study is the use of sodium chloride as transport medium, which might result in a slightly lower detection rate than with the use of viral transport medium. However, this weakness should be equal to both sampling sites. A strength of the study is that it is as far as possible reflecting real life in an emergency department. The sampling is performed by any of the ordinary staff when the patient presents in the acute phase of the disease. Thus the method could be easily implemented in other emergency departments.
An important challenge when interpreting PCR results from infected individuals is whether asymptomatic viral carriage was present before the current disease yielding positive irrelevant PCR results. Studies on viral carriage in persons without respiratory symptoms point at a more prevalent asymptomatic viral carriage in children than adults. In one study oropharyngeal samples were obtained from 232 children 10–15 years old, healthy enough to be in school, and 31/50 (62%) of those who had a positive PCR results were asymptomatic [Citation24]. In another study 31/127 (24%) children and 5/238 (2%) adults without respiratory symptoms yielded a positive PCR respiratory agent result on combined nasopharynx/oropharynx nylon flocked swabs [Citation25]. Further on asymptomatic respiratory tract viral carriage was found by PCR on nasopharyngeal, oropharyngeal or nasal wash samples in 32/450 (3%) adults without symptoms of infection [Citation26].
In conclusion, we have found that oropharyngeal sampling could be a patient comfortable alternative to the normally performed nasopharyngeal sampling when diagnosing respiratory viruses. Combined nasopharyngeal and oropharyngeal samples, however, enhance the diagnostic yield.
Acknowledgements
The authors thank all the participating patients and the staff at the Malmö Emergency Department and at the Department of Microbiology, Laboratory Medicine, Lund for their effort when the study was performed. The authors also thank Linus Jengard for valuable discussions regarding statistics.
Disclosure statement
The authors declare that they have no conflict of interest.
References
- McGeer A, Green KA, Plevneshi A, et al. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis. 2007;45:1568–1575.
- Benet T, Sanchez Picot V, Messaoudi M, et al. Microorganisms associated with pneumonia in children <5 years of age in developing and emerging countries: The GABRIEL pneumonia multicenter, prospective, case-control study. Clin Infect Dis. 2017;65:604–612.
- Feng L, Li Z, Zhao S, et al. Viral etiologies of hospitalized acute lower respiratory infection patients in China, 2009-2013. PloS ONE. 2014;9:e99419.
- Yu X, Lu R, Wang Z, et al. Etiology and clinical characterization of respiratory virus infections in adult patients attending an emergency department in Beijing. PloS ONE. 2012;7:e32174.
- Ostby AC, Gubbels S, Baake G, et al. Respiratory virology and microbiology in intensive care units: a prospective cohort study. Apmis. 2013;121:1097–1108.
- Brittain-Long R, Westin J, Olofsson S, et al. Access to a polymerase chain reaction assay method targeting 13 respiratory viruses can reduce antibiotics: a randomised, controlled trial. BMC Medicine 2011;9:44.
- Falsey AR, Murata Y, Walsh EE. Impact of rapid diagnosis on management of adults hospitalized with influenza. Arch Intern Med. 2007;167:354–360.
- Hansen KB, Westin J, Andersson LM, et al. Flocked nasal swab versus nasopharyngeal aspirate in adult emergency room patients: similar multiplex PCR respiratory pathogen results and patient discomfort. Infect Dis (London). 2016;48:246–250.
- Tunsjo HS, Berg AS, Inchley CS, et al. Comparison of nasopharyngeal aspirate with flocked swab for PCR-detection of respiratory viruses in children. APMIS. 2015;123:473–477.
- Blaschke AJ, Allison MA, Meyers L, et al. Non-invasive sample collection for respiratory virus testing by multiplex PCR. J Clin Virol. 2011;52:210–214.
- Hernes SS, Quarsten H, Hamre R, et al. A comparison of nasopharyngeal and oropharyngeal swabbing for the detection of influenza virus by real-time PCR. Eur J Clin Microbiol Infect Dis. 2013 ;32:381–385. Mar
- Yan XL, Li YN, Tang YJ, et al. Clinical characteristics and viral load of respiratory syncytial virus and human metapneumovirus in children hospitaled for acute lower respiratory tract infection. J Med Virol. 2017;89:589–597.
- Nilsson AC, Brytting M, Serifler F, et al. Longitudinal clearance of seasonal influenza A viral RNA measured by real-time polymerase chain reaction in patients identified at a hospital emergency department. Scand J Infect Dis. 2010;42:679–686.
- Spencer S, Gaglani M, Naleway A, et al. Consistency of influenza A virus detection test results across respiratory specimen collection methods using real-time reverse transcription-PCR. J Clin Microbiol. 2013;51:3880–3882.
- Lieberman D, Lieberman D, Shimoni A, et al. Identification of respiratory viruses in adults: nasopharyngeal versus oropharyngeal sampling. JCM 2009;47:3439–3443.
- Hernes SS, Quarsten H, Hagen E, et al. Swabbing for respiratory viral infections in older patients: a comparison of rayon and nylon flocked swabs. Eur J Clin Microbiol Infect Dis. 2011;30:159–165.
- Daley P, Castriciano S, Chernesky M, et al. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. JCM 2006;44:2265–2267.
- Kim C, Ahmed JA, Eidex RB, et al. Comparison of nasopharyngeal and oropharyngeal swabs for the diagnosis of eight respiratory viruses by real-time reverse transcription-PCR assays. PloS ONE. 2011;6:e21610.
- Spyridaki IS, Christodoulou I, de Beer L, et al. Comparison of four nasal sampling methods for the detection of viral pathogens by RT-PCR-A GA(2)LEN project. J Virol Methods. 2009;156:102–106.
- Macfarlane P, Denham J, Assous J, et al. RSV testing in bronchiolitis: which nasal sampling method is best?. Arch Dis Child. 2005;90:634–635.
- Walsh P, Nguyen TA, Higashida K, et al. Do infants and toddlers prefer nasal swabs or washes for specimen collection?. Pediatr Infect Dis J. 2010;29:1156–1157.
- Lieberman D, Lieberman D, Shimoni A, et al. Pooled nasopharyngeal and oropharyngeal samples for the identification of respiratory viruses in adults. Eur J Clin Microbiol Infect Dis. 2010;29:733–735.
- Ison MG, Lee N. Influenza 2010-2011: lessons from the 2009 pandemic. Cleve Clin J Med. 2010;77:812–820.
- Nilsson AC, Persson K, Bjorkman P, et al. Frequent detection of respiratory agents by multiplex PCR on oropharyngeal samples in Swedish school-attending adolescents. Scand J Infect Dis. 2012 ;44:393–397. May
- Self WH, Williams DJ, Zhu Y, et al. Respiratory viral detection in children and adults: comparing asymptomatic controls and patients with community-acquired pneumonia. J Infect Dis. 2016;213:584–591.
- Lieberman D, Shimoni A, Shemer-Avni Y, et al. Respiratory viruses in adults with community-acquired pneumonia. Chest 2010;138:811–816.