Abstract
Background and aims: Sharing of unsterile injection equipment contributes to transmission of hepatitis C virus (HCV), HIV and hepatitis B virus (HBV) among people who inject drugs (PWID) but is largely preventable through needle exchange programmes (NEP). Sweden has been one of the last countries in Europe to scale up access to NEP for PWID, who consequently have high prevalence rates of HCV and HIV. The aim of the study was to investigate demographic and drug-related determinants of injection risk behaviours, sharing of needle/syringe and paraphernalia, and patterns of change over time in subgroups of PWID participating in the Stockholm NEP.
Methods: The Stockholm NEP started in 2013 as the first formal NEP in the region. A total of 2860 PWID were included in this prospective open cohort study. The association between demographic and drug-related determinants and injection risk behaviours were analysed at baseline and followed up at 6, 12, 24, 36 and 48 months post-enrolment.
Results: The following factors were associated with high levels of injection risk behaviours at inclusion: female gender, homelessness, low education level, younger age, amphetamine use, not in opioid substitution therapy (OST), being HIV negative and being HCV positive. We found an overall significant decrease in injection risk behaviours over time among participants. Not previously investigated in depth, we found that subgroups of participants varied in degrees of injection risk behaviour reduction over time and that women decreased injection risk behaviours faster than men. Enrolment in OST, HIV positive and age <25 years at inclusion were not associated with a decrease in injection risk behaviours over time.
Conclusions: In this prospective cohort study over 4 years, we found that NEP participation was associated with a significant decrease in injection risk behaviours.
Introduction
There are approximately 15.6 million people who inject drugs (PWID) worldwide [Citation1]. Sharing of unsterile injection equipment is the leading cause of hepatitis C virus (HCV), HIV and hepatitis B virus (HBV) infections among PWID. Around 2.8 million PWID are living with HIV and 6.1 million with HCV worldwide [Citation1,Citation2]. In Sweden, the population of PWID is estimated at around 8000 with an HCV prevalence of about 60% [Citation3–6]. Approximately 2000 incident HCV cases are reported annually in a total population of 10 million in Sweden, of which half are confirmed as associated with injecting drug use [Citation7]. In 2016, the World Health Organization (WHO) presented global goals for eliminating HBV and HCV by 2030, including recommendations for the scale-up of harm reduction programmes [Citation8]. Several focus areas were proposed, including a thorough investigation of the efficacy of prevention programmes.
Several reviews have concluded that needle exchange programmes (NEP) are effective in reducing injection risk behaviours, HIV and HCV among PWID [Citation9–14]. Sweden has historically been slow to introduce NEP for political reasons; however, the country is currently noticing a fast expansion with 17 of 21 regions providing NEP with the Stockholm NEP as the largest programme [Citation4]. There is still a lack of knowledge on predictors for favourable long-term outcomes following initiation and participation in a NEP [Citation15]. Factors such as early drug debut, being a woman and being homeless are associated with a higher degree of injection risk behaviours but less is known about the impact of NEP for different PWID subgroups over time [Citation7]. Most studies investigating NEP have used cross-sectional study designs, which does not allow for studying changes in risk behaviours over time [Citation16]. Cohort studies using a longitudinal design have shown a general reduction in self-reported injection risk behaviours among PWID but have seldom been designed to study subgroup variations in outcomes [Citation13,Citation14]. Furthermore, these studies mostly included participants with previous experience of NEP at baseline, compared change in injection risk behaviours between street-based PWID with and without access to NEP and often had a limited follow-up time [Citation17–30].
The aim of this study was to investigate injection risk behaviours, i.e. level of receptive sharing of needle and/or syringe (needle/syringe) and paraphernalia (i.e. cookers/filters/water) at inclusion and over a four-year follow-up time in a cohort of PWID enrolled in the Stockholm NEP 2013–2018. More specifically, the aim was to investigate demographic characteristics and determinants of injection risk behaviours at inclusion into the NEP and trajectories of change in injection risk behaviours over time for the following subgroups: gender, age at inclusion, age of drug debut, drug at last injection, level of education, living situation past three months and HCV and HIV status. A further aim was to investigate differences in baseline characteristics and injection risk behaviours, dependent on time point of admission in the programme.
Methods
Study setting
The Stockholm NEP first opened in April 2013 and has been the only official site during the study period. The NEP offers sterile injection equipment (i.e. needle/syringe and paraphernalia), testing for hepatitis A, HBV, HCV and HIV at inclusion [Citation4,Citation5]. Vaccination, risk-reducing counselling, wound care, treatment for HCV and HIV, referral to social services and dependency disorder units, including opioid substitution treatment (OST), are provided at the Stockholm NEP. The NEP is organized by physicians and nurses specialized in infectious diseases and psychiatry/dependency disorders, a counsellor and midwives [Citation4,Citation5].
Study participants
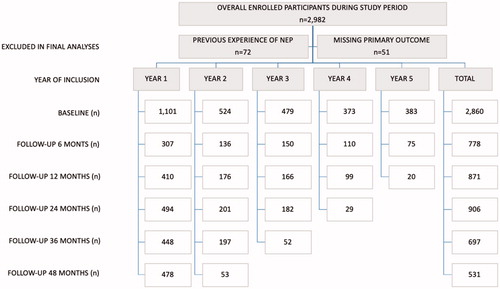
Swedish legislations for NEP participation require the following: 1) age ≥18 years (≥20 years before March 2017); 2) active injecting drug use and 3) provision of valid identification [Citation31]. All participants enrolled in the NEP since the start in April 2013 were eligible for inclusion but excluded if 1) ever enrolled in another formal NEP in Sweden and 2) missing data on primary outcome (sharing of needle/syringe or paraphernalia) at inclusion. Between 8 April 2013 and 7 April 2018, a total of 2982 PWID were enrolled of which 122 were excluded in the final analyses ().
At inclusion, participants were informed that data provided in the NEP would be used in future research projects. The study was performed in accordance with the Helsinki declaration and was approved by The Regional Ethical Review Board in Stockholm (Dnr: 2013/495-31/3 and 2015/1374-32).
Recruitment
At first registration in the NEP, all participants underwent a face-to-face interview performed by personnel not related to the research team, containing 34 questions on baseline demographics (country of birth, level of education, marital status, housing conditions and employment), past and ongoing drug use, contacts with health care services, social services, prison and prohibition services. Participants were registered with their unique Swedish personal identity number, while those without were given a unique reserve number. All data were registered in the InfCare Needle Syringe Program database [Citation4,Citation5].
Measures of risk behaviours
The following definitions of self-reported injection risk behaviours were used as main outcomes: 1) receptive sharing of needle/syringe the past month (yes/no) and 2) receptive sharing of paraphernalia the past month (yes/no). These behaviours were measured at inclusion with follow-up points set at 6 months (±2 months) for an early follow-up and thereafter at 12 (±3), 24 (±5), 36 (±5) and 48 (±5) months. The ±3–5 months’ span was set to allow for flexibility with follow-up visits and to minimize respondents being lost to follow-up. All participants were followed to their last registered interview within the above-defined time frames and study period.
Determinants for injection risk behaviours over time
Based on previous research [Citation4,Citation5,Citation7], the following determinants were used in the statistical analysis: 1) gender (woman, man); 2) age at inclusion (<25, 25–34, >34 years); 3) age at injecting drug debut (0–19, ≥20 years); 4) duration of injecting drug use (0–5, ≥6 years); 5) drug used at last injection (heroin, amphetamine, methylphenidate, buprenorphine, other); 6) self-reported living situation past 3 months (homeless, not homeless [co-habitant/own housing contract]); 7) level of education (partial or full elementary, upper secondary/vocational, university); 8) OST (yes, no); 9) HIV status (negative, positive); 10) HBV status (negative, positive, cleared infection, vaccinated); and 11) HCV status (negative, positive/viremic).
Statistical methods
Descriptive characteristics for the study population at baseline are presented as frequencies and percentages. Chi-square or Fisher’s exact two-tailed tests were used for categorical variables and the Wilcoxon rank-sum test for continuous values to test for differences in baseline characteristics.
The odds of the two of injection risk behaviours at inclusion and five follow-up points, given the 11 determinants previously described, were modelled using generalized estimating equation (GEE) regression models [Citation32]. Given the longitudinal nature of the data, GEE regression models were used to account for the potential dependence in the injection risk behaviours within participants over time. Based on those models, we first report the associations between the single determinants and the odds of the injection risk behaviours at baseline (). We then report the relative change in odds of the injection risk behaviours over the five follow-up points for all determinant categories, together with a p value testing for an overall change over time (Supplementary Table 1). Results are reported as odds ratios (ORs) with corresponding 95% confidence intervals (CIs). All reported p values are two-sided, and p values <.05 were considered as statistically significant. Data were analysed using Stata 15 (StataCorp, College Station, TX, USA) and JMP 13.0® (SAS Institute Inc., Cary, NC, USA).
Table 1. Baseline characteristics at inclusion for the included study population and injection risk behaviours (2013–2018, N = 2860).
Table 2. Adjusted odds ratios (aORs) for sharing needle/syringe and paraphernalia during the past month at inclusion, (2013–2018, N = 2860).
Results
shows baseline demographic characteristics of NEP participants. Of 2860 respondents included in the final analysis, 24% were women and over two-thirds were Swedish born. The median age at inclusion was 35.5 years for women and 38.8 years for men (interquartile range (IQR) 26–46 and IQR 29–48 years, respectively). Almost half (45.2%) had started injecting drugs before age 20 (median age for both gender was 20 years) and 57.5% versus 30% reported amphetamine and heroin at injecting drug debut. Overall, 60% had injected drugs for 10 years or more, with a median duration of 14 years (IQR 5–24). The median duration for injecting drug use was 15 years (IQR 6–15) and 10 years (IQR 4–21) for men and women, respectively. Almost half (43.6%) reported having children and 13.8% were fully or part-time employed. Nearly 80% (79.1%) reported having a home compared to 20.7% reporting being homeless. More than one in five (22.1%) had been in custody or prison the past 12 months and almost three-quarters (72.2%) reported prior experience of custody or prison. Overall, a majority (71.2%) were enrolled in the treatment or support services, with 56.2% stating regular contact with the social services, 33.8% treatment for substance use disorders and 9.1% receiving OST.
Nearly half of the respondents (48.6%) reported daily injection and 35.1% injected several times per day (data not shown). Twenty-nine per cent had shared needle/syringe the past month, 34.1% had shared paraphernalia and one-fifth (19.8%, 567/2860) had engaged in both injection risk behaviours. Laboratory-confirmed prevalence of HIV, HBV and HCV (viremic) was 4.9%, 1.4% and 55%, respectively.
Socio-demographic determinants for injection risk behaviours at inclusion
Almost half of all women had shared needle/syringe (42.9%) and paraphernalia (50.4%) the past month compared to 24.7% and 28.9% among men (). When adjusting for confounders, women were twice as likely as men to share needle/syringe (aOR 1.95; 95% CI 1.61, 2.35) and paraphernalia (aOR 2.41; 95% CI 1.99, 2.91) ().
Those being homeless reported higher odds for sharing both needle/syringe and paraphernalia compared to those not being homeless (aOR 1.48; 95% CI 1.20, 1.82 and aOR 1.50; 95% CI 1.23, 1.83). Upper secondary/vocational education was associated with 20% lower odds for sharing paraphernalia (aOR 0.80; 95% CI 0.67, 0.96) compared to those with lower education level. Equally, those with a university education showed 33% lower odds for sharing paraphernalia compared to those with a partial or full elementary education (aOR 0.67; 95% CI 0.47, 0.94). At registration, PWID ≥34 years showed 35% lower odds for sharing needle/syringe compared to their younger peers (aOR 0.65; CI 95% 0.53, 0.80) ().
Drug-related determinants for injection risk behaviours at inclusion
We found no association between age of injecting drug debut and injection risk behaviours (). However, participants injecting amphetamine were 33% and 58% more likely to share needle/syringe (aOR 1.33; 95% CI 1.09, 1.61) and paraphernalia (aOR 1.58; 95% CI 1.31, 1.91) compared to participants who mainly injected heroin. Being enrolled in OST was associated with lower levels of sharing needle/syringe (aOR 0.66; CI 95% 0.46, 0.95) and sharing paraphernalia (aOR 0.35; CI 95% 0.23, 0.51).
Blood-borne-related determinants for injection risk behaviours at inclusion
HIV-positive participants reported lower levels of injection risk behaviours compared to HIV negative participants: needle/syringe (aOR 0.56; CI 95% 0.35, 0.92) and paraphernalia (aOR 0.62; CI 95% 0.40, 0.96). The reverse association was found among HCV positive participants who reported higher risk levels (aOR 1.31; CI 95% 1.10, 1.58 for needle/syringe and aOR 1.41; CI 95% 1.18, 1.68 for paraphernalia) ().
Changes in injection risk behaviours over time in the NEP
Overall changes in injection risk behaviours for participants over time, at different time points following inclusion in the NEP, are displayed in .
Figure 2. Changes in injection risk behaviours (sharing needle/syringe and paraphernalia) following inclusion in the NEP. Odds ratio (OR) at inclusion is set at 1 as reference value. N = 2860 at inclusion. p values represent changes in injection risk behaviours over the whole follow-up time.
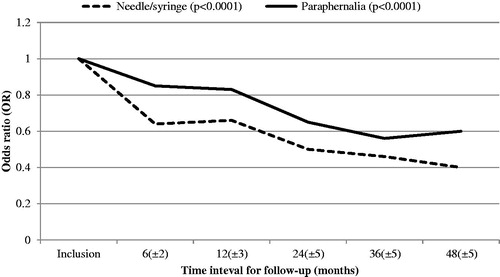
Over time, decreases regarding both sharing needle/syringe and paraphernalia (p < .0001 and p < .0001) were observed among participants, with reductions noticed already after 6 months. When performing subanalyses, all determinants, except injecting methylphenidate/buprenorphine/other, enrolment in OST, HIV or HBV positive, age <24 years at inclusion, were associated with decreased injection risk behaviours over the whole study period (Supplementary Table 1).
Women’s risk in sharing paraphernalia decreased significantly already after 6 months participation in the NEP (OR 0.48; CI 95% 0.35, 0.67) compared to men, for whom significant risk reduction occurred after 12 months (OR 0.74; CI 95% 0.61, 0.90). However, even though both women and men significantly decreased injection risk behaviours over time, women consistently reported higher risk levels at each time point compared to men (Supplementary Table 2).
There were significant differences in injection risk behaviours trajectory over time regarding OST. Those not enrolled in OST showed a reduction in odds for sharing paraphernalia already within six months (OR 0.79; CI 95% 0.66, 0.94) as well as over the whole period (p < .0001), contrary to those enrolled in OST showing no significant changes over time (Supplementary Table 1).
Differences in injection risk behaviours and baseline demographics over time of inclusion in the NEP
No major differences in injection risk behaviours were found for gender when comparing baseline characteristics at different years of inclusion in the NEP. However, participants enrolled year five in comparison with year one were found to be younger (age ≤24, 17.0% vs 10.4% p = .001) reported later injecting drug debut (age ≥20 at injecting drug debut, 63.2% vs 50.6%, p < .001) and shorter duration of injecting drug use (duration of injecting drug use 0–2 years, 25.6% vs 9.4%, p < .001). The proportion of participants injecting heroin increased from 38.0% at year one to 46.2% at year five (p < .01). Furthermore, a higher proportion of participants (6.9%) were HIV positive year one compared to 1.3% year five, p < .01, and the proportion of HCV positive was reduced from 61.1% to 42.0%, p < .001. Over time, a higher proportion of participants were HBV negative (33.2% vs 53.3%, p < .001), while a lower proportion had serological markers for previous (cleared) HBV infection (40.8% vs 21.9%, p < .001). The level of HBV vaccinated at inclusion remained stable around 20%.
Lost to follow-up analysis
To investigate possible bias in our results due to lost to follow-up, we analysed possible differences in baseline characteristics and injection risk behaviours between those followed to the last point of measure (48 months) and those lost to follow-up at 48 months. There were no differences regarding baseline characteristics or injection risk behaviours among those still in the NEP at 48 months compared to those lost to follow-up (p = .53 for sharing needle/syringe and p = .11 for sharing paraphernalia, respectively).
Discussion
The aim of this study was to investigate injection risk behaviours at inclusion and over time in the Stockholm NEP. At inclusion, women, participants using amphetamine, PWID not in OST, being homeless, and HIV negative and HCV positive participants reported elevated levels of injection risk behaviours. In line with previous prospective cohort studies, we found a general reduction in injection risk behaviours among participants over time. However, not previously shown, we also found evidence for differences in injection risk behaviour trajectories between time points and over time, for PWID subgroups participating in the NEP.
In line with previous research, women compared to men were twice as likely to share needle/syringe as well as paraphernalia at inclusion [Citation33–35]. However, women’s injection risk behaviours more than halved already within the first six months after inclusion, compared to men showing a decrease in risk at 12-month follow-up, a decrease that continued over time for both subgroups. This rapid change, already within the first year, is consistent with data from a longitudinal study from Baltimore, USA, where the greatest risk reduction was noticed within the first year of follow-up, although this study did not investigate injection risk behaviour changes within subgroups [Citation36]. To our knowledge, our observed rapid risk reduction among women is somewhat unique, corroborating the validity in calls for gender-disaggregated data for a deeper understanding of the specific needs for women who inject drugs regarding efficacious harm reduction interventions [Citation37–39].
Our results were in line with previous research showing that PWID not being homeless reported lower levels of injection risk behaviours compared to homeless PWID [Citation7,Citation40,Citation41]. Participants injecting amphetamine were more likely to share injection equipment compared to those injecting heroin. Being enrolled in OST was associated with lower injection risk behaviours at inclusion, also shown in previous reviews [Citation9,Citation11]. However, OST participants did not reduce their injection risk behaviours over time, possibly indicating a floor effect where OST participants showed a relatively low level of injection risk behaviours already at inclusion in the NEP and with less room left for further reduction. In our data, we found no association between young age at injecting drug debut and increased injection risk behaviours as previously shown in other studies [Citation7,Citation34,Citation42].
HCV positive PWID reported higher injection risk behaviours at inclusion compared to those uninfected. However, over time both groups reduced their risk-taking, with a 40% risk reduction for sharing needle/syringe already within the first six months. Despite this risk reduction among HCV negative participants, the HCV incidence in the Stockholm NEP has previously been high at 22/100 person-years [Citation5]. This indicates that a significant reduction in injection risk behaviours through NEP alone is not enough to prevent the spread of HCV among PWID, mirroring previous studies that highlight the need of combined interventions of NEP, OST and scale-up of HCV treatment to combat the HCV epidemic [Citation6,Citation10,Citation11,Citation43–45]. The reverse injection risk behaviour relationship found among HIV-positive participants, where HIV-positive status was associated with lower injection risk behaviours, may be explained by changes in risk-taking following being diagnosed with HIV and possibly also by the required mandatory contact with HIV health care.
Analyses based on time of inclusion in the NEP showed that entrants at year five were overall younger and reported a shorter duration of injecting drug use, indicating that the NEP gradually has become more efficient in reaching a broader population of PWID. Previous research has shown that young injectors are at higher risk for sharing injection equipment and are more exposed for contracting HCV and HIV [Citation7,Citation46–48]. Thus, reaching PWID at an early stage of their injecting drug use provides an opportunity for early prevention and treatment interventions.
We also found that the proportion of participants injecting heroin increased between year one and five at inclusion. However, this difference disappeared when controlling for age, as participants was younger at year five. Nevertheless, previous studies have shown that heroin compared to amphetamine as preferred drug has effect on injection risk behaviours [Citation7,Citation48]. This may guide more customized prevention interventions for people who use amphetamine in the future, but also highlights the importance and benefit of providing OST for participants injecting heroin. Platt et al. concluded that OST was associated with a 50% reduction in the HCV transmission rate and that NEP alone was less effective with only a 21% reduction of the transmission rate. Combined OST/NEP on the other hand was associated with a 74% reduction of the HCV transmission rate [Citation11].
Strengths and weaknesses
All data, except status of HIV and hepatitis, were based on self-reports. The risk for underreporting injection risk behaviours cannot be ruled out and might thus represent recall bias or social desirability response bias. A lack of awareness of HIV and hepatitis status that might affect injection risk behaviours was not considered a confounding factor in this study since all participants were tested and informed about their actual serostatus at follow-up [Citation4].
Furthermore, Swedish NEP legislation requires participants to provide identification documents before admission, an age ≥18 (previously ≥20 years) and mandatory testing for HIV and hepatitis. These procedures may have deterring effects on some PWID, making them reluctant to participate. On the other hand, these requirements provide opportunities to collect data on an individual and programme-level basis and thus prospectively follow injection risk behaviours, serostatus and related factors over time.
Due to Swedish legislations, the Stockholm NEP is a needle/syringe exchange, not a needle/syringe provision programme. However, the dispensing policy is not on a 1:1 basis and has gradually been adapted to a more need-based distribution. In the WHO strategy for HCV elimination, a goal of >300 needle/syringes per PWID per year is proposed for an effective prevention of HIV and hepatitis transmission [Citation49]. With a suboptimal NEP due to syringe dispensing policies, low syringe coverage and reach and limited hours of operations may result in insufficient reduction of injection risk behaviour and a continuous spread of HIV and hepatitis [Citation50–53].
Finally, since the Stockholm NEP first started in 2013, all included participants with inclusion data (2013–2018) could not be followed up over 48 months. Hence, the sample size was rather small in some subgroup follow-up analyses, calling for cautious interpretation of these results. In future studies, a longer follow-up time will allow us to draw more firm conclusions regarding injection risk behaviours over time, based on a larger number of participants.
Conclusions
This longitudinal cohort study design provided opportunities to investigate NEP effects on injection risk behaviours in PWID subgroups by demographic and drug-related determinants at different time points, and over time. Despite differences in injection risk behaviours at inclusion and despite decreases in absolute risk levels for subgroups over time, those with high risk at inclusion continued to show higher levels of injection risk behaviours throughout the follow-up period. Women, participants using amphetamine, HCV positive, HIV negative, homeless PWID and those not in OST may benefit from more specifically tailored interventions within the NEP, to further reduce injection risk behaviours and the consequent risk of blood-borne infections.
Supplemental Material
Download MS Word (32.9 KB)Acknowledgements
The help from the clinical staff at the Stockholm NEP in terms of data collection is highly appreciated.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Degenhardt L, Peacock A, Colledge S, et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Global Health. 2017;5:e1192–e207.
- Grebely J, Larney S, Peacock A, et al. Global, regional, and country-level estimates of hepatitis C infection among people who have recently injected drugs. Addiction (Abingdon, England). 2018;114:150–166.
- Socialstyrelsen. [The National Board of Health and Welfare]. En uppskattning av omfattningen av injektionsmissbruket i Sverige [An estimate of the extent of the injection drug abuse in Sweden]. Stockholm, Sweden: Socialstyrelsen; 2013. p. 16.
- Kaberg M, Hammarberg A, Lidman C, et al. Prevalence of hepatitis C and pre-testing awareness of hepatitis C status in 1500 consecutive PWID participants at the Stockholm needle exchange program. Infectious Diseases (London, England). 2017;49:728–736.
- Kaberg M, Naver G, Hammarberg A, et al. Incidence and spontaneous clearance of hepatitis C virus (HCV) in people who inject drugs at the Stockholm Needle Exchange-Importance for HCV elimination. J Viral Hepat. 2018;25:1452–1461.
- Fraser H, Martin NK, Brummer-Korvenkontio H, et al. Model projections on the impact of HCV treatment in the prevention of HCV transmission among people who inject drugs in Europe. J Hepatol. 2017;68:402–411.
- Karlsson N, Santacatterina M, Kall K, et al. Risk behaviour determinants among people who inject drugs in Stockholm, Sweden over a 10-year period, from 2002 to 2012. Harm Reduct J. 2017;14:57.
- WHO. Combating hepatitis B and C to reach elimination to 2030. 2016.
- MacArthur GJ, van Velzen E, Palmateer N, et al. Interventions to prevent HIV and Hepatitis C in people who inject drugs: a review of reviews to assess evidence of effectiveness. Int J Drug Policy. 2014;25:34–52.
- Palmateer NE, Taylor A, Goldberg DJ, et al. Rapid decline in HCV incidence among people who inject drugs associated with national scale-up in coverage of a combination of harm reduction interventions. PloS One. 2014;9:e104515.
- Platt L, Minozzi S, Reed J, et al. Needle syringe programmes and opioid substitution therapy for preventing hepatitis C transmission in people who inject drugs. Cochrane Database Syst Rev. 2017;9:CD012021.
- Larney S, Peacock A, Leung J, et al. Global, regional, and country-level coverage of interventions to prevent and manage HIV and hepatitis C among people who inject drugs: a systematic review. Lancet Global Health. 2017;5:e1208–e1220.
- Fernandes RM, Cary M, Duarte G, et al. Effectiveness of needle and syringe Programmes in people who inject drugs - an overview of systematic reviews. BMC Public Health. 2017;17:309.
- Palmateer N, Kimber J, Hickman M, et al. Evidence for the effectiveness of sterile injecting equipment provision in preventing hepatitis C and human immunodeficiency virus transmission among injecting drug users: a review of reviews. Addiction (Abingdon, England). 2010;105:844–859.
- Grebely J, Bruneau J, Lazarus JV, et al. Research priorities to achieve universal access to hepatitis C prevention, management and direct-acting antiviral treatment among people who inject drugs. Int J Drug Policy. 2017;47:51–60.
- Aspinall EJ, Nambiar D, Goldberg DJ, et al. Are needle and syringe programmes associated with a reduction in HIV transmission among people who inject drugs: a systematic review and meta-analysis. Int J Epidemiol. 2014;43:235–248.
- Hagan H, Thiede H. Changes in injection risk behavior associated with participation in the Seattle needle-exchange program. J Urban Health. 2000;77:369–382.
- Monterroso ER, Hamburger ME, Vlahov D, et al. Prevention of HIV infection in street-recruited injection drug users. The Collaborative Injection Drug User Study (CIDUS). J Acquired Immune Deficiency Syndromes (1999). 2000;25:63–70.
- Bluthenthal RN, Kral AH, Gee L, et al. The effect of syringe exchange use on high-risk injection drug users: a cohort study. AIDS (London, England). 2000;14:605–611.
- Cox GM, Lawless MC, Cassin SP, et al. Syringe exchanges: a public health response to problem drug use. Ir Med J. 2000;93:143–146.
- Gibson DR, Brand R, Anderson K, et al. Two- to sixfold decreased odds of HIV risk behavior associated with use of syringe exchange. J Acquired Immune Deficiency Syndromes (1999). 2002;31:237–242.
- Hart GJ, Carvell AL, Woodward N, et al. Evaluation of needle exchange in central London: behaviour change and anti-HIV status over one year. AIDS (London, England). 1989;3:261–265.
- Huo D, Bailey SL, Garfein RS, et al. Changes in the sharing of drug injection equipment among street-recruited injection drug users in Chicago, Illinois, 1994–1996. Subst Use Misuse. 2005;40:63–76.
- Ouellet L, Huo D, Bailey SL. HIV risk practices among needle exchange users and nonusers in Chicago. J Acquired Immune Deficiency Syndromes (1999). 2004;37:1187–1196.
- Schoenbaum EE, Hartel DM, Gourevitch MN. Needle exchange use among a cohort of injecting drug users. AIDS (London, England). 1996;10:1729–1734.
- van Ameijden EJ, Coutinho RA. Maximum impact of HIV prevention measures targeted at injecting drug users. AIDS (London, England). 1998;12:625–633.
- van den Hoek JA, van Haastrecht HJ, Coutinho RA. Risk reduction among intravenous drug users in Amsterdam under the influence of AIDS. Am J Public Health. 1989;79:1355–1357.
- Vertefeuille JM, Tun W, Huettner S, et al. Decline in self-reported high-risk injection-related behaviors among HIV-seropositive participants in the Baltimore needle exchange program. AIDS Behav. 2000;4:381–388.
- Vlahov D, Junge B, Brookmeyer R, et al. Reductions in high-risk drug use behaviors among participants in the Baltimore needle exchange program. J Acquired Immune Deficiency Syndromes Human Retrovirol. 1997;16:400–406.
- Wood E, Tyndall MW, Spittal PM, et al. Factors associated with persistent high-risk syringe sharing in the presence of an established needle exchange programme. AIDS (London, England). 2002;16:941–943.
- Ökad tillgänglighet till sprututbytesverksamheter i Sverige [Increased Access to Needle Exchange Programs in Sweden] (Prop. 2016/17:15). Stockholm (Sweden): Parliament Documents; 2016.
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130.
- Montgomery SB, Hyde J, De Rosa CJ, et al. Gender differences in HIV risk behaviors among young injectors and their social network members. Am J Drug Alcohol Abuse. 2002;28:453–475.
- Evans JL, Hahn JA, Page-Shafer K, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study). J Urban Health. 2003;80:137–146.
- Sherman SG, Latkin CA, Gielen AC. Social factors related to syringe sharing among injecting partners: a focus on gender. Subst Use Misuse. 2001;36:2113–2136.
- Huo D, Ouellet LJ. Needle exchange and injection-related risk behaviors in Chicago: a longitudinal study. J Acquired Immune Deficiency Syndromes (1999). 2007;45:108–114.
- Iversen J, Page K, Madden A, et al. HIV, HCV, and health-related harms among women who inject drugs: implications for prevention and treatment. J Acquired Immune Deficiency Syndromes (1999). 2015;69:S176–S181.
- Malinowska-Sempruch K. What interventions are needed for women and girls who use drugs? A global perspective. J Acquired Immune Deficiency Syndromes (1999). 2015;69:S96–S97.
- El-Bassel N, Strathdee SA. Women who use or inject drugs: an action agenda for women-specific, multilevel, and combination HIV prevention and research. J Acquired Immune Deficiency Syndromes (1999). 2015;69:S182–S190.
- Strathdee SA, Hallett TB, Bobrova N, et al. HIV and risk environment for injecting drug users: the past, present, and future. Lancet (London, England). 2010;376:268–284.
- Rhodes T, Singer M, Bourgois P, et al. The social structural production of HIV risk among injecting drug users. Social Sci Med (1982). 2005;61:1026–1044.
- Booth RE, Kwiatkowski CF, Mikulich-Gilbertson SK, et al. Predictors of risky needle use following interventions with injection drug users in Ukraine. Drug Alcohol Depend. 2006;82:S49–S55.
- Palmateer NE, Hutchinson SJ, Innes H, et al. Review and meta-analysis of the association between self-reported sharing of needles/syringes and hepatitis C virus prevalence and incidence among people who inject drugs in Europe. Int J Drug Policy. 2013;24:85–100.
- Martin NK, Hickman M, Hutchinson SJ, et al. Combination interventions to prevent HCV transmission among people who inject drugs: modeling the impact of antiviral treatment, needle and syringe programs, and opiate substitution therapy. Clin Infect Dis. 2013;57:S39–S45.
- Turner KM, Hutchinson S, Vickerman P, et al. The impact of needle and syringe provision and opiate substitution therapy on the incidence of hepatitis C virus in injecting drug users: pooling of UK evidence. Addiction (Abingdon, England). 2011;106:1978–1988.
- Vorobjov S, Des Jarlais DC, Abel-Ollo K, et al. Socio-demographic factors, health risks and harms associated with early initiation of injection among people who inject drugs in Tallinn, Estonia: evidence from cross-sectional surveys. Int J Drug Policy. 2013;24:150–155.
- Folch C, Casabona J, Espelt A, et al. High prevalence and incidence of HIV and HCV among new injecting drug users with a large proportion of migrants–is prevention failing? Subst Use Misuse. 2016;51:250–260.
- Palmateer N, Hutchinson S, McAllister G, et al. Risk of transmission associated with sharing drug injecting paraphernalia: analysis of recent hepatitis C virus (HCV) infection using cross-sectional survey data. J Viral Hepat. 2014;21:25–32.
- WHO. Global Health Sector strategy on viral hepatitis 2016–2021, towards ending viral hepatitis. Geneva; 2016.
- Kerr T, Small W, Buchner C, et al. Syringe sharing and HIV incidence among injection drug users and increased access to sterile syringes. Am J Public Health. 2010;100:1449–1453.
- Bluthenthal RN, Ridgeway G, Schell T, et al. Examination of the association between syringe exchange program (SEP) dispensation policy and SEP client-level syringe coverage among injection drug users. Addiction (Abingdon, England). 2007;102:638–646.
- Bluthenthal RN, Anderson R, Flynn NM, et al. Higher syringe coverage is associated with lower odds of HIV risk and does not increase unsafe syringe disposal among syringe exchange program clients. Drug Alcohol Depend. 2007;89:214–222.
- Sherman SG, Patel SA, Ramachandran DV, et al. Consequences of a restrictive syringe exchange policy on utilisation patterns of a syringe exchange program in Baltimore, Maryland: implications for HIV risk. Drug Alcohol Rev. 2015;34:637–644.