Abstract
A child with pre-B acute lymphoblastic leukaemia (ALL) developed fatal encephalitis associated with human coronavirus OC43 (HCoV-OC43). During chemotherapy the child had a persistent HCoV-OC43 respiratory infection and later developed progressive encephalitis. Cerebrospinal fluid was negative for pathogens including HCoV-OC43, but a brain biopsy was HCoV-OC43-positive by metagenomic next-generation sequencing.
Introduction
Coronaviruses are able to cause infections in humans, mammals and birds [Citation1]. Human coronavirus OC43 (HCoV-OC43) is a relatively common cause of respiratory tract infections in children. Most infected cases have a mild upper or, more rarely, lower respiratory tract infection. In contrast to respiratory syncytial virus (RSV), HCoV-OC43 is more frequently diagnosed in children with underlying disease [Citation2].
The identification of aetiological agents of encephalitis is challenging. In a 39-long time-series at our hospital the aetiology of childhood encephalitis remained unknown in nearly 40% of cases, a situation which has not improved substantially over time [Citation3]. We here report a fatal case of encephalitis associated with coronavirus OC43 in a child with acute lymphoblastic leukaemia (ALL). We discuss the role of metagenomics and indication of brain biopsy for the purpose.
Case report
Clinical presentation and management
A 9-month-old infant presented with hyperleukocytosis, anaemia and thrombocytopenia at the emergency ward at the Astrid Lindgren Children’s Hospital, Stockholm, Sweden in May 2017. The patient’s medical history included a vaginal delivery at term, birthweight 3,350 kg and no postnatal complications. The child was vaccinated according to the national guidelines for childhood immunizations with the addition of BCG as the child was of African origin. The child had a normal growth and development prior to admission.
After a thorough medical work up, the infant was diagnosed with pre-B acute lymphoblastic leukaemia (ALL) without CNS involvement. Genetic characterisation of the ALL clone showed a genetic aberration, MLL-rearrangement, which confers a poor prognosis and requires high-dose chemotherapy. Induction therapy was initiated according to the NOPHO Infant ALL protocol and at day 29, the child was in complete remission with a bone marrow minimal residual disease (MRD) with < 0.1% leukemic blasts. Consolidation therapy according to the NOPHO2008 high risk arm [Citation4] started in June 2017 and was scheduled for 7 intensive multimodal chemotherapy blocks over 5–7 days every 3–4 weeks depending on length of neutropenia and toxicity. During consolidation therapy, the child suffered from culture-verified septicaemia caused by Klebsiella pneumoniae on three occasions and with Chryseobacterium spp. and Staphylococcus epidermidis once each. However, between the blocks the child recovered and was in a fairly good clinical condition and in a sustained MRD negative remission.
In October 2017 during the last planned block therapy, the mother reported that the child had upper airway symptoms accompanied with fatigue and recurring high fever. Extensive microbiological sampling for bacteria, virus and fungi only revealed a positive PCR for HCoV-OC43 in a nasopharyngeal swab sample. Respiratory samples were tested for influenza A, influenza B and respiratory syncytial virus using the Simplexa™ Flu A/B & RSV Direct Kit and 11 additional respiratory viruses, including HCoV-OC43, using the SeeGene Allplex™ Respiratory Panel 2 and 3. Several additional respiratory samples collected in November and December 2017 were positive for HCoV-OC43 with cycle threshold values (Ct) ranging from 22–24. In conjunction with treatment protocol, a lumbar puncture for cytology was performed mid-December which revealed pleocytosis with 12 PMN cells/µl and 5 mono/µl and normal cerebrospinal fluid (CSF) protein levels. This was interpreted as a non-specific inflammatory reaction. Upon re-puncture a few days later the cell count had further increased to 34 PMN/µl while CSF was negative for bacteria, herpes simplex 1 and 2, varicellae-zoster virus, enterovirus and HCoV-OC43. A CT scan performed at the time showed no intracranial pathology and a pulmonary X-ray was normal. The child’ s general condition stabilised, and the child was discharged at the end of Dec 2017 with a diagnosis of persistent coronavirus infection in the upper airways. A month later, i.e. the end of Jan 2018, the child was readmitted due to altered behaviour and myoclonic seizures involving the abdominal wall.
Diagnostic assessment
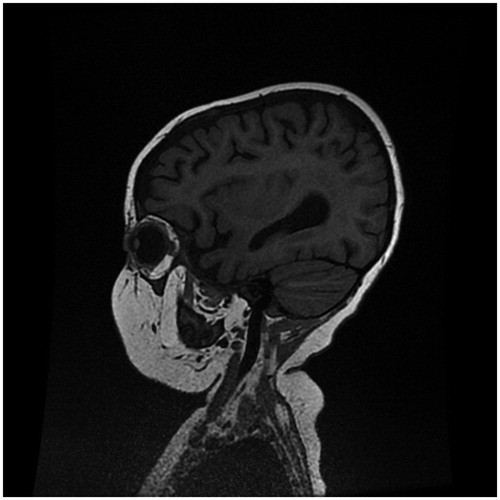
On admission EEG was inconclusive showing only a slow general activity without epileptic foci while MRI brain at end of January showed slightly increased T2-signal in white matter near lateral ventricles, and bilaterally in thalami and pons (). Spectroscopy showed low levels of lactate. Analyses of CSF for infectious causes of meningoencephalitis were all negative, including PCR for herpes simplex virus type 1 & 2, varicella zoster virus, enterovirus, Epstein-Barr virus, cytomegalovirus, human herpesvirus 6, parvovirus B19, adenovirus, influenza and cryptococcal-Ag and Borrelia burgdorferi IgM/IgG.
However, there were highly elevated levels of neuronal damage markers such as tau and neurofilament-light in CSF. The patient was severely immunocompromised with no Th-lymphocytes (B-CD3 + CD4+) or Tc-lymphocytes (B-CD3 + CD8+) in peripheral blood. Based on our knowledge of one previous report of HCoV-OC43 associated with fatal encephalitis in an immunocompromised child [Citation5] we tested a CSF sample collected Feb 13 for HCoV-OC43 by real-time PCR, but the result was negative. We then used metagenomic next-generation sequencing on the Ion Torrent platform on CSF but could not identify any infectious agent. We finally decided to do a brain biopsy on 18 Mars 2018, both for PAD (Malignant disease? Inflammatory disease?) and metagenomic analysis. The neurosurgeon opened a 3x3 cm bone flap and continued to open the dura and arachnoidea and found an atrophied brain and took a biopsy on 1 cm depth, corresponding to white matter. The brain biopsy was grinded and total nucleic acids were extracted with MagLEAD (PSS) together with a negative sample that followed the same process. The library construction was performed automatically by the AB Library builder system (Thermo Fisher Scientific). The Ion Xpress™ Plus Library Kit was used to generate barcoded Ion fragment libraries of DNA and Ion Total RNA-seq kit was used for the barcoded RNA libraries. The template preparation and sequencing was performed by the Ion Chef and Ion S5 XL instrument (Thermo Fisher Scientific), respectively.
Raw reads were filtered to eliminate low-complexity reads using a DUST filter. Remaining reads were classified using the software Kraken, towards a database of all RefSeq genomes for pathogens as well as the human genome. Reads that were not classified by Kraken were BLASTed towards the viral genomes, in order to find highly divergent viral sequences. Putative positive hits were validated by mapping reads to the respective reference genome and by BLAST searches against NCBI nucleotide collection (nr) to eliminate false-positives.
The metagenomic analysis generated 24 million reads of which approximately 300,000 reads were classified as HCoV-OC43. These reads were assembled into a near complete (99.7%) HCoV-OC43 genome with a mean coverage of 1500x. To verify the finding, we performed an accredited in-house HCoV-OC43 real-time PCR [Citation6] on the brain tissue, which confirmed the presence of HCoV-OC43 RNA with a cycle threshold value of 26. The PAD on the brain biopsy showed no ALL on histomorphology or with PAX5 immunochemistry. During the period October 2017 – March 2018 a total of fourteen respiratory samples continued to be positive for HCoV-OC43 RNA.
Therapeutic intervention
There is no established treatment for HCoV-OC43 infections. There is, however, preclinical and case-series support for the HIV protease inhibitor lopinavir boosted by ritonavir (LPV/r) in the treatment of MERS-CoV [Citation7] and an ongoing randomised controlled trial in Saudi-Arabia is recruiting patients with MERS-CoV [Citation8]. There are also media reports about LPV/r being tried in the ongoing outbreak of 2019-nCoV in China. Since MERS-CoV and HCoV-OC43 are members of the same virus family, the patient was started on experimental treatment with LPV/r mixture 0,2 ml/kg x 2 po. We measured the concentration of lopinavir after 7 days and the trough concentration in serum was 16 µmol/L.
Follow-up and outcome
Unfortunately, the patient’s condition did not improve over 10 days of antiviral treatment. Instead, the neurological status deteriorated; on day 10 of treatment the patient had Glasgow Coma Scale 3 without sedation in respirator, myoclonus, absent cough on tracheal suctioning, but had a positive corneal reflex. After a multidisciplinary conference and an ethic discussion which included the child’s parents it was decided to discontinue all intensive care. The patient died soon after. The mother gave oral consent to this case report.
Conclusions
To determine the cause of infectious encephalitis is challenging, especially in immunocompromised patients, in whom encephalitis can have unexpected causes. Furthermore, the aetiological agent sometimes may only be detected in CSF early in infection or not at all. Therefore analysis of brain tissue can have a higher likelihood of finding the causative agent as illustrated in this and a previous report [Citation9]. However, brain biopsy has several limitations: there is risk for bleeding and neurological damage due to the trauma [Citation10,Citation11]. There are also difficulties to access deeper affected areas. Although we do not have definitive proof, we find it likely that our patient had a persistent HCoV-OC43 infection that with time gave the child a fatal encephalitis. We feel that in selected cases of encephalitis where a definite diagnose is needed for further management, there is clearly a place for brain biopsy and metagenomics. Negative results by routine diagnostics and metagenomics on a brain biopsy may indicate a non-infectious cause. However, negative results on metagenomics do not exclude infections; in part because metagenomics still have moderate sensitivity compared with specific molecular diagnostics of infectious agents, i.e. compared with nucleic acid amplification techniques such as PCR. The main advantage of metagenomics is that it is unbiased and therefore can detect unexpected pathogens, such as the detection of HCoV-OC43 in our case.
Acknowledgements
Maria Lind-Karlberg at the Public Health Agency of Sweden for metagenomics workup and bioinformatic analysis.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Spaan W, Cavanagh D, Horzinek MC. Coronaviruses – structure and genome expression. J Gen Virol. 1988;69(12):2939–2952.
- Lee J, Storch GA. Characterization of human coronavirus OC43 and human coronavirus NL63 infections among hospitalized children <5 years of age. Pediatr Infect Dis J. 2014;33(8):814–820.
- Wickstrom R, et al. Review of the aetiology, diagnostics and outcomes of childhood encephalitis from 1970 to 2009. Acta Paediatrica. 2017;106:463–469.
- Toft N, Birgens H, Abrahamsson J, et al. Results of NOPHO ALL2008 treatment for patients aged 1–45 years with acute lymphoblastic leukemia. Leukemia. 2018;32(3):606–615.
- Morfopoulou S, Brown JR, Davies EG, et al. Human coronavirus OC43 associated with fatal encephalitis. N Engl J Med. 2016;375(5):497–498.
- Tiveljung-Lindell A, Rotzén-Östlund M, Gupta S, et al. Development and implementation of a molecular diagnostic platform for daily rapid detection of 15 respiratory viruses. J Med Virol. 2009;81(1):167–175.
- Chan JF-W, Yao Y, Yeung M-L, et al. Treatment with lopinavir/ritonavir or interferon-beta 1b improves outcome of MERS-CoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212(12):1904–1913.
- Arabi YM, Alothman A, Balkhy HH, et al. Treatment of middle east respiratory syndrome with a combination of lopinavir-ritonavir and interferon-beta1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19(1):81.
- Brown JR, Bharucha T, Breuer J. Encephalitis diagnosis using metagenomics: application of next generation sequencing for undiagnosed cases. J Infect. 2018;76(3):225–240.
- Dhawan S, He Y, Bartek J, et al. Comparison of frame-based versus frameless intracranial stereotactic biopsy: systematic review and meta-analysis. World Neurosurg. 2019;127:607.e4–616.e4.
- Riche M, Amelot A, Peyre M, et al. Complications after frame-based stereotactic brain biopsy: a systematic review. Neurosurg Rev. [cited 2020 Jan 4]. DOI:10.1007/s10143-019-01234-w